Introduction
Risk factors concerning orthodontic miniscrew implants have not been adequately assessed. In this systematic review, we summarize the knowledge from published clinical trials regarding the failure rates of miniscrew implants used for orthodontic anchorage purposes and identify the factors that possibly affect them.
Methods
Nineteen electronic databases and reference lists of included studies were searched up to February 2011, with no restrictions. Only randomized controlled trials, prospective controlled trials, and prospective cohort studies were included. Study selection and data extraction were performed twice. Failure event rates, relative risks, and the corresponding 95% confidence intervals were calculated. The random-effects model was used to assess each factor’s impact. Subgroup and meta-regression analyses were also implemented.
Results
Fifty-two studies were included for the overall miniscrew implant failure rate and 30 studies for the investigation of risk factors. From the 4987 miniscrew implants used in 2281 patients, the overall failure rate was 13.5% (95% confidence interval, 11.5-15.8). Failures of miniscrew implants were not associated with patient sex or age and miniscrew implant insertion side, whereas they were significantly associated with jaw of insertion. Certain trends were identified through exploratory analysis; however, because of the small number of original studies, no definite conclusions could be drawn.
Conclusions
Orthodontic miniscrew implants have a modest small mean failure rate, indicating their usefulness in clinical practice. Although many factors seem to affect their failure rates, the majority of them still need additional evidence to support any possible associations.
Orthodontic miniscrew implants have been popularized because of their simplicity of placement and removal, low cost, and minimal need for patient compliance. The value of miniscrew implants is in the prerequisite that they remain relatively stationary in the bone, their ability to increase anchorage capacity, and the absence of adverse effects or complications that could endanger health or treatment outcome.
Their clinical effectiveness lies in their ability to maintain close bone contact, thus resisting reactive orthodontic forces. The term orthodontic anchorage describes the nature and degree of resistance to displacement provided by an anatomic unit and is crucial for the maximization of tooth movement and the minimization of undesired effects. Conventional orthodontic anchorage often results in anchorage loss, which is considered a significant potential side effect of orthodontic mechanotherapy. More than 2 mm of anchorage loss can undermine treatment efficacy, especially in critical situations. Anchorage reinforcement with miniscrew implants is associated with 2.4 mm less anchorage loss compared with conventional anchorage means. In addition, miniscrew implants seem to be more effective in supporting anchorage when they are used in the mandible, between the second premolars and the first molars, when 2 miniscrew implants are inserted into a patient’s jaw, when they are directly connected, when they are used in adults, and when treatment lasts more than 12 months.
Several complications during the use of miniscrew implants have been reported. Lack of initial stability is often observed in case of inadequate cortical bone thickness. If insertion results in injury to adjacent structures (periodontal ligament, tooth root, nerves, blood vessels, or sinus), the miniscrew implants should be removed and inserted in a different location. This rarely affects tooth prognosis. Inflammation and infection of the tissues around miniscrew implants are not rare events and are generally regarded as significant problems. However, antibiotics are not needed, except for extreme symptoms. The choice of insertion site is essential to prevent soft-tissue irritation or inflammation, and firm attached gingiva is usually advocated rather than movable mucosa. Other measures to prevent hypertrophy of the peri-implant soft tissues include the use of healing cap abutments over the miniscrew implants or complete coverage of the miniscrew implant with the oral mucosa, while the wires or attachments connected to the miniscrew implant head pass through the mucosa. Loss of miniscrew implant stability is an irreversible phenomenon that can be attributed to inflammation or bone remodeling. Meticulous oral hygiene is critical, and 0.2% chlorhexidine mouth rinses or dental floss dipped in 2% chlorhexidine solution can be used to prevent and control any inflammation or infection. Miniscrew implants have been demonstrated to be not entirely stable, due to the lack of proper osseointegration. Movement of 1 to 1.5 mm can be expected. As a general rule, it is advisable to leave a clearance of 2 mm from the roots of teeth, nerves, and so on, especially when the miniscrew implants are inserted in interdental areas.
Failure of miniscrew implants results in inability to act as stationary anchors that negate reaction forces and necessitates their removal or replacement. According to a Kaplan-Meier analysis of miniscrew implants, most failures occurred within 100 to 150 days after loading with orthodontic forces. At this point, a change in the treatment plan might be, at least in some patients, difficult or impossible. Fracture of miniscrew implants can be experienced during their removal, especially if the diameter of the head or the thread is too narrow, but this has little effect on the outcome. Difficulty in miniscrew implant removal is occasionally observed, mainly due to partial osseointegration that usually takes place after their prolonged use and is associated with high removal torque values.
The factors that are possibly associated with the success (or failure) of orthodontic miniscrew implants have been assessed in clinical studies and partially in previous systematic reviews. However, these systematic reviews included only a limited number of studies, they assessed only a few factors qualitatively, and they also included anchorage-reinforcement means other than miniscrew implants, such as mini-implants (implants with a diameter greater than 2 mm) and miniplates.
The aims of this meta-analysis were to summarize in an evidence-based manner the current knowledge from published controlled and uncontrolled prospective clinical trials regarding the failure rates of miniscrew implants used for orthodontic anchorage purposes and to identify any significant risk factors possibly affecting these failure rates.
Material and methods
This meta-analysis was based on the guidelines provided by the PRISMA statement and the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0).
Systematic searches were conducted for published, unpublished, and ongoing studies up to February 2011. The databases searched are shown in Appendix 1 . The reference lists of the articles eligible for inclusion were also manually reviewed. Systematic reviews and meta-analyses relevant to this study were identified, and their reference lists were also scanned for additional trials. In addition, conference abstracts were searched, and we inquired about their current status. Articles published in journals, dissertations, and conference proceedings were located from several electronic databases by using an appropriately adjusted search strategy for each database ( Appendix 1 ).
No restrictions were applied concerning publication year, language, or status. Grey literature was not excluded from our search. If additional information was needed, the authors were contacted. Translations were arranged for 6 articles (1 in Italian, 3 in Chinese, and 2 in Portuguese ).
Selection of studies
Randomized controlled trials are study designs of original research providing the highest level of evidence and thus are considered the gold standard for assessing the efficacy of various orthodontic treatment approaches, including miniscrew implants. Nevertheless, nonrandomized studies can also contribute to the investigation of miniscrew implant failures. Thus, the eligible studies included randomized controlled trials, prospective controlled clinical trials, and prospective cohort studies investigating the success and survival of miniscrew implants used for orthodontic anchorage reinforcement in patients of any age and sex. Studies on mini-implants (implants with a diameter greater than 2 mm) and miniplates were excluded. All other clinical and nonclinical study designs were excluded. Studies including 2 or more anchorage means were included if the miniscrew implant data could be isolated.
Two review authors (S.N.P. and I.P.Z.) screened all titles and abstracts obtained from the database searches. Duplicate records, such as published articles also presented in conferences, studies with multiple publications, and dissertations also published as journal articles, were excluded. The same authors reviewed the full texts of the potentially relevant titles and abstracts against the inclusion criteria. The eligibility of the trials was assessed independently, and any differences were settled by consensus after consulting the third author (M.A.P.), and the level of agreement between the 2 review authors was assessed with the Cohen kappa statistic.
Data extraction and management
Two authors (S.N.P. and I.P.Z.) independently extracted study characteristics and outcomes from the included studies using predefined data extraction forms. Any disagreements were resolved by discussion with the third author (M.A.P.). The Cohen kappa statistic was used to assess the agreement between the 2 review authors. Miniscrew implant failure counts were extracted as a binary outcome and converted to failure event rates. The primary outcome was the overall miniscrew implant failure rate, and associated factors were the secondary outcomes. Risk factors were assessed by comparing 2 or more event rates provided by a study.
Assessment of risk of bias of the studies
Again, 2 review authors (S.N.P. and I.P.Z.) assessed independently the risk of bias of the included randomized controlled trials using the Cochrane Collaboration’s tool for assessing risk of bias by means of the specific software RevMan (version 5.1), as guided by the Cochrane Handbook for Systematic Reviews of Interventions . The following domains were considered: (1) adequate sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) incomplete outcome data, (5) selective outcome reporting, and (6) other sources of bias. For all included trials, the risk of bias for each domain was judged as low risk, high risk, or unclear risk. Each randomized controlled trial was assigned an overall risk of bias in terms of low risk (low for all key domains), high risk (high for ≥1 key domain), and unclear risk (unclear for ≥1 key domain).
The risk of bias of the included nonrandomized prospective clinical trials was independently assessed by the same authors using the Newcastle-Ottawa Scale, as suggested by the Cochrane Handbook for Systematic Reviews of Interventions . The Newcastle-Ottawa Scale assigns a maximum of 4 points for selection, a maximum of 2 points for comparability, and a maximum of 3 points for exposure or outcome, resulting in a maximum score of 9 points for the highest quality. Study quality was thus judged as high (7-9 points), medium (4-6 points), or low (1-3 points), similarly to other published material.
Any disagreement on the risk of bias assessment was resolved after consulting the third author (M.A.P.). The level of agreement between the 2 investigators was assessed with the Cohen kappa statistic.
Data analysis
Data were summarized and considered suitable for pooling if the corresponding studies used similar interventions in the same way and reported similar outcomes. Failures of miniscrew implants were expressed as event rates and their corresponding 95% confidence intervals (CIs), and they were reported as a percentage failure rate. For every factor, only studies that provided failure data for 2 or more factor-based groups of miniscrew implants were used (eg, female and male patients regarding the factor “sex”). Comparisons among the various groups of risk factors were performed with subgroup analyses, whereas random-effects meta-regression (method of moments) was used to investigate between-studies heterogeneity for continuous variables. For safety reasons, a minimum of 5 studies was deemed to be adequate for the meta-analysis of possible risk factors affecting miniscrew implant failures. Comparisons with less than 5 studies were regarded as exploratory analyses and must be viewed with caution until additional research becomes available. Stratified analyses were also performed on the basis of jaw of insertion; ie, the maxilla (including the palate) and the mandible. Significant associations that included 3 or more studies were also quantified by calculating the relative risk. A random-effects model as proposed by DerSimonian and Laird rather than a fixed-effect model was used as the primary method to estimate all pooled estimates, since this model takes into account the heterogeneity, and it can be considered more conservative in terms of the significance intervals. In addition, since the observed effect was expected to differ across studies because of sample and implementation differences, the use of this model seemed more appropriate.
The pooled estimate of the individual miniscrew implant failure rates weighted by the random-effects model and the corresponding 95% CIs were used to construct a forest plot according to the procedures of the statistical software Comprehensive Meta-Analysis (version 2.0; Biostat, Englewood, NJ). Subgroup analyses were conducted according to the comprehensive meta-analysis ( www.meta-analysis.com ) procedures. All P values were 2-sided with a level of significance of α = 0.05, except for the heterogeneity tests (α = 0.10).
Assessment of heterogeneity
Heterogeneity and possible causes for it were assessed by visual inspection of the forest plots, by the Q statistic and the corresponding P value, and by means of the I² index, which is an indicator of true heterogeneity in percentages. A value of 0% indicates no observed heterogeneity, and larger values show increasing heterogeneity (with 25% indicating low, 50% moderate, and 75% high heterogeneity).
Assessment of publication bias
Publication bias was initially evaluated through visual inspection of funnel plot asymmetry, which, however, should be seen as a means of examining small study effects and not as tool to diagnose specific types of bias.
In addition, publication bias was tested statistically by using the test of Begg and Mazumdar (rank correlation method) and the test of Egger et al (weighted regression). When it was deemed appropriate, the trim-and-fill procedure of Duval and Tweedie was used to compute an estimate of the effect size after the publication bias had been taken into account (adjusted effect size).
Sensitivity analysis
Since the main outcome of many studies with limited numbers of miniscrew implants was the assessment of treatment effects (cephalometric changes) rather than the miniscrew implant failure rate, a separate analysis was conducted including only studies with 100 or more miniscrew implants that could be considered more relevant to the investigation of risk factors.
Results
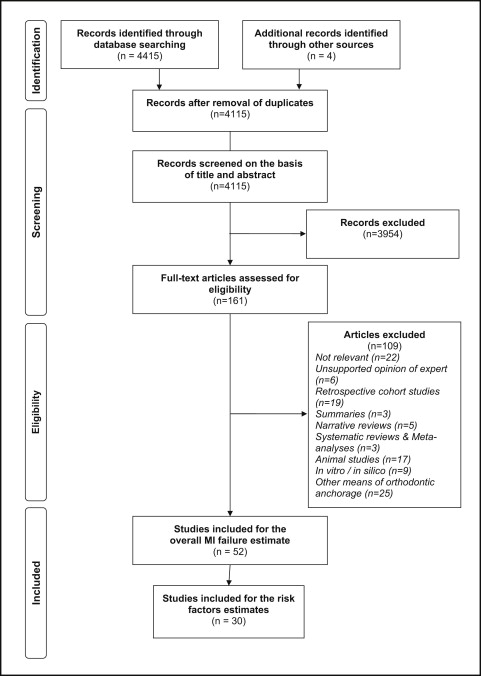
The flow information of the search and selection of studies is shown in Figure 1 . Among the 4419 initially identified relevant articles, 4115 unique citations remained after removal of duplicates. A total of 3954 articles were excluded on the basis of title and abstract, and 109 articles were excluded on the basis of their full texts. The sums of all excluded articles (n = 4063) with the corresponding reasons for exclusion are presented in Table I . Thus, 52 studies remained for qualitative and quantitative synthesis. These were categorized according to study design into 5 randomized controlled trials, 8 prospective controlled clinical trials, 27 prospective cohort studies, and 12 studies with unclear designs that were considered through detailed reading to be prospective cohort studies. The complete list of the included studies is in Table II .
| Reason for exclusion | Excluded articles |
|---|---|
| Investigations not relevant to the subject of this study | 2287 |
| Unsupported opinion of expert | 727 |
| Editors’ choices | 22 |
| Replies to the author or editor | 8 |
| Interviews | 6 |
| Commentaries | 18 |
| Book or conference abstracts | 95 |
| Summaries | 60 |
| Cross-sectional surveys | 6 |
| Retrospective clinical trials | 11 |
| Narrative reviews | 140 |
| Systematic reviews | 23 |
| Meta-analyses | 3 |
| Animal studies | 325 |
| In-vitro or in-silico studies | 128 |
| Studies on molecular biology, histology, or genetics | 179 |
| Human studies that refer to patients receiving anchorage reinforcement by means other than miniscrew implants | 25 |
| Total | 4063 |
| Trial | Author | Design | Setting | MIs (n) | Type | Dimensions | Success criteria | Failure rate | Handling of failure | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Per patient (per jaw) | Diameter (mm) | Length (mm) | ||||||||
| 1 | Aboul-Ela | RCT b | University | 26 | 2 (2) | AbsoAnchor (Dentos, Daegu, Korea) | 1.3 | 8 | Stability | 7.7 | Repositioned |
| 2 | Alves M Jr et al | PCS | University | 41 | 2/3 (2/3) | (INP, São Paulo, Brazil) | 1.4/2 | 6/8 | NR | 14.6 | New † |
| 3 | Apel et al | PCCT b | University | 76 | 2/4 (2) | Tomas-pin (Dentaurum, Ispringen, Germany) | 1.6 | 8 | Stability/infection | 10.5 | Excluded |
| 4 | Baek et al | PCS ∗ | NR | 109 | 1/2 (1/ 2) | THOplant (Biomaterials Korea, Seoul, South Korea) | 2 | 5 | Stability/infection/Tx completion | 24.8 | New |
| 5 | Basha et al | RCT a | University | 14 | 2 (2) | SS | 1.3 | 8 | Stability | 28.6 | New |
| 6 | Bayat and Bauss | PCS ∗ | Private | 110 | 1-4 (1/2) | LOMAS (Mondeal Medical Systems, Tuttlingen, Germany) | 2 | 7/9/11 | Stability/infection | 18.2 | NR |
| 7 | Berens et al | PCS ∗ | Private | 239 | 1-3 (1/2) | AbsoAnchor (Dentos, Daegu, Korea)/Dual-Top (Jeil Medical, Seoul, Korea) | 1.4/1.8/2 | NR | Stability | 15.1 | Rescrewed/excluded |
| 8 | Blaya et al | PCS | University/private | 30 | 1 (1) | Sin Implant System (São Paulo, Brazil) | 1.2 | 10 | Stability | 0.0 | NR |
| 9 | Brandão and Mucha | PCS | University | 40 | 4 (2) | Ortoimplante Básicos (Conexão, Arujá, Brazil) | 1.5 | 9 | Stability | 0.0 | NR |
| 10 | Chaddad et al | PCS ∗ | NR | 32 | 2/4 (2) | C-Implant (Implantium, Seoul, Korea)/Dual-Top (Jeil Medical, Seoul, Korea) | 1.4-2 | 6-10 | Stability/infection/Tx completion | 12.5 | NR |
| 11 | Cheng et al ∗∗ | PCS | University | 92 | NR | Leibinger (Freiburg, Germany)/Mondeal (Tuttlingen, Germany) | 2 | 5-15 | Stability/infection/Tx completion | 8.7 | NR |
| 12 | El-Beialy et al | PCS ∗ | University | 40 | NR | AbsoAnchor (Dentos, Daegu, Korea) | 1.2 | 8 | Stability | 17.5 | Excluded |
| 13 | Freudenthaler et al | PCS ∗ | NR | 15 | NR | Leibinger (Freiburg, Germany) | 2 | 13 | Stability/soft-tissue problem | 6.7 | Excluded/new |
| 14 | Fritz et al | PCS ∗ | University | 36 | NR | (Jeil Medical, Seoul, Korea) | 1.4/1.6/2 | 6/8/10 | Stability | 30.6 | NR |
| 15 | Garfinkle et al | PCCT b | University/private | 82 | 4/8 (4) | Osteomed (Addison, Tex) | 1.6 | 6 | Stability/Tx completion | 19.5 ˆ | NR |
| 16 | Gelgor et al | PS | University | 25 | 1 (1) | IMF Stryker (Leibinger, Germany) | 1.8 | 14 | Stability | 0.0 | NR |
| 17 | Gelgor et al | PCS ∗ | NR | 40 | 1 (1) | IMF Stryker (Leibinger, Germany) | 1.8 | 14 | Stability | 0.0 | NR |
| 18 | Hedayati et al | PCCT a | University | 27 | 3 (1/2) | Orthognathic screws | 2 | 9/11 | Stability | 18.5 | Repositioned |
| 19 | Herman et al | PCS | NR | 49 | 1/2 (1/2) | Ortho Implant (IMTEC, Ardmore, Okla), Sendax MDI | 1.8 | 6/8/10 | Stability | 40.8 | New/excluded |
| 20 | Justens and De Bruyn | PCS | University | 50 | NR | Dual-Top (Jeil Medical, Seoul, Korea) | 1.8/2 | 8/10 | Stability/Tx completion | 34.0 ∗∗∗ | New |
| 21 | Kim et al | PCS ∗ | University | 197 | 1 (1) | KLS-Martin (Jacksonville, Fla)/Orthoplant | 1.5/2 | 5 | Stability | 9.2 | New |
| 22 | Kim et al | PCS | University | 50 | 2 (2) | C-Implant (Implantium, Seoul, Korea) | 1.8 | 8.5 | Stability | 4.0 | New |
| 23 | Kuroda et al | PCS ∗ | University | 216 | NR | AbsoAnchor (Dentos, Daegu, Korea)/(Gebrüder Martin, Tuttlingen, Germany) | 1.3/1.5 | 6-12 | Tx completion | 16.2 | NR |
| 24 | Lee et al | PCS ∗ | NR | 260 | 2 (2) | C-Implant (Implantium, Seoul, Korea) | 1.8 | 8.5 | NR | 8.5 | NR |
| 25 | Lehnen et al | RCT b | NR | 60 | 2 (2) | Tomas-pin (Dentaurum, Ispringen, Germany) | 1.6 | 8 | NR | 11.7 | Excluded |
| 26 | Liou et al | PCS | NR | 32 | 2 (2) | Leibinger (Freiburg, Germany) | 2 | 17 | Stability | 0.0 | NR |
| 27 | Liu et al | RCT a | NR | 68 | 2 (2) | (Cibei, Ningbo, China) | 1.2 | 8 | Stability | 11.8 | New |
| 28 | Luzi et al | PCS | University | 140 | NR | Aarhus Mini-Implants (Medicon, Germany) | 1.5/2 | 9.6/11.6 | Stability/Tx completion | 15.7 ∗∗∗ | Excluded |
| 29 | Maddalone et al | PCCT a | NR | 25 | NR | (3M Unitek, Monrovia, Calif) | 1.8 | 8 | Stability | 8.0 | NR |
| 30 | Miyazawa et al | PCS | University | 44 | NR | (Jeil Medical, Seoul, Korea) | 1.6 | 8 | Tx completion | 9.1 | NR |
| 31 | Motoyoshi et al | PCS | University | 124 | 1-4 (1/2) | ISA orthodontic implants (BIODENT, Tokyo, Japan) | 1.6 | 8 | Stability | 14.5 | NR |
| 32 | Motoyoshi et al | PCS | University | 169 | 1-4 (1/2) | (BIODENT, Tokyo, Japan) | 1.6 | 8 | Stability/Tx completion | 14.8 | NR |
| 33 | Motoyoshi et al | PCS | University | 87 | NR | ISA orthodontic implants (BIODENT, Tokyo, Japan) | 1.6 | 8 | Stability/Tx completion | 12.6 | NR |
| 34 | Motoyoshi et al | PCS | University | 209 | 1-4 (1/2) | ISA orthodontic implants (BIODENT, Tokyo, Japan) | 1.6 | 8 | Stability/Tx completion | 11.5 | NR |
| 35 | Motoyoshi et al | PCS | University | 148 | NR | ISA orthodontic implants (BIODENT, Tokyo, Japan) | 1.6 | 8 | Stability | 9.5 | Excluded |
| 36 | Oh et al | PCS | University | 78 | NR | AbsoAnchor (Dentos, Daegu, Korea)/Osteomed (Dallas, Tex) | 1.2/NR | NR | NR | 10.3 ‡ | New |
| 37 | Park et al | PCS | University | 227 | NR | Stryker Leibinger (Freiburg, Germany)/Osteomed (Addison, Tex)/AbsoAnchor, Dentos, Daegu, Korea/KLS-Martin (Jacksonville, Fla) | 1.2/2 | 4-15 | Stability/Tx completion | 8.4 | New |
| 38 | Park et al | PCCT a | University | 46 | 2/4 (2) | AbsoAnchor (Dentos, Daegu, Korea)/ Osteomed (Addison, Tex)/Leibinger (Freiburg, Germany) | 1.2 | 6/8 ¶ | Tx completion | 13 | New |
| 39 | Polat-Ozsoy et al | PCS | University | 22 | 2 (2) | AbsoAnchor (Dentos, Daegu, Korea) | 1.2 | 6 | Stability/infection | 13.6 § | New |
| 40 | Shi et al | PCCT a | University | 28 | 2 (2) | MAS (Titanium Biological Products, Xi’an Bang, China) | 1.5 | 8 | NR | 10.7 | New |
| 41 | Suzuki and Suzuki | PCS | University | 280 | NR | Sistema Nacional de Implantes (São Paulo, Brazil)/ACR Mini-Implant (BioMaterials Korea, Seoul, Korea) | 1.5 | 6/8 | NR | 6.8 | NR |
| 42 | Thiruvenkatachari et al | PCS | University | 18 | 1/2 (1) | Titanium microimplant | 1.3 | 8 | Stability | 0.0 | NR |
| 43 | Türköz et al | PCS | University | 112 | 1/2 (1/2) | AbsoAnchor (Dentos, Daegu, Korea) | 1.4 | 7 | Stability | 22.3 | NR |
| 44 | Upadhyay et al | RCT a | University | 72 | 4 (2) | Modified Ti fixation screws | 1.3 | 8 | Stability | 6.9 | New |
| 45 | Upadhyay et al | PCCT a | University | 30 | 2 (2) | Modified Ti fixation screws | 1.3 | 8 | Stability | 10.0 | New |
| 46 | Upadhyay et al | PCS | University | 46 | 2 (2) | Ti mini-implants | 1.3 | 8 | NR | 4.3 | New |
| 47 | Viwattanatipa et al | PCS | University | 97 | 2 (2) | Osteomed (Addison, Tex) | 1.2 | 8-12 | Mobility/dislodgement/infection | 33.0 | NR |
| 48 | Wang et al | PCS ∗ | University | 77 | NR | MIA system (Dentos, Daegu, Korea)/SDIA system (Zhejiang Cixi Oral Biomaterials, China) | 1.2/2 | 7/8 | NR | 7.8 | NR |
| 49 | Wang et al | PCS | University | 298 | 2 (2) | Micro-planting nail (North Medical, Ningbo, China) | 1.6 | 11 | Stability/Tx completion | 22.8 | Excluded |
| 50 | Wiechmann et al | PCS | NR | 133 | AbsoAnchor (Dentos, Daegu, Korea)/Dual-Top (Jeil Medical, Seoul, Korea) | 1.2/1.6 | 5-10 | Stability/Tx completion/infection | 23.3 | NR | |
| 51 | Wilmes et al | PCCT a | NR | 10 | 1 (1) | Dual-Top (Jeil Medical, Seoul, Korea) | 2 | 10 | NR | 0.0 | NR |
| 52 | Wu et al | PCS | University | 414 | NR | AbsoAnchor (Dentos, Daegu, Korea)/LOMAS (Mondeal Medical Systems)/A1 (Bio-ray, Syntec Scientific, Chang Hua, Taiwan)/(Mondel Medical System) | 1.1-1.7/2 | 7-13 | Stability/Tx completion | 10.1 | NR |
∗ Reinstalled MI failures were not considered.
‡ Four MIs were omitted because they were intentionally moved to another location.
§ One miniscrew was replaced due to root proximity and was also classified as a failure.
¶ No information was given for the Leibinger implants.
∗∗ Only MI data are reported from the various anchorage-reinforcement methods used by Cheng et al
∗∗∗ Including failed miniscrews, which did not need to be replaced to complete treatment.
Study characteristics and risk of bias
The characteristics of the 52 original studies included in the meta-analysis are summarized in Table II . Thirty-eight studies (73%) took place in a university setting; in 14 studies (27%), the setting was either private or not reported. The mean number of miniscrew implants used per study was 96, and the number of miniscrew implants per patient ranged from 1 to 4. Miniscrew implants with various brand names were used in the studies, with thread diameters from 1.1 to 2.0 mm and thread lengths from 5.0 to 15.0 mm. In most studies, a miniscrew implant was defined as successful when it had no mobility or withstood orthodontic force application during treatment. However, in 9 studies (17%), success or failure was not defined, although the success rates were reported. In 26 studies (50%), no details were given regarding how the miniscrew implant failures were handled: ie, whether the failed implant was excluded, replaced with another, or repositioned in another location.
In all 5 randomized controlled trials, the allocation concealment was assessed as unclear because of insufficient information, and hints of other forms of bias were also found. For example, blinding of participants and personnel was inadequate in 3 of the 5 randomized controlled trials ( Appendix 2 ). With regard to the 8 prospective controlled clinical trials, 1 was of high quality (Newcastle-Ottawa Scale score higher than 6), and the other 7 were of medium quality ( Appendix 3 ). None of the remaining 39 prospective cohort studies was high quality, although 24 were medium quality (Newcastle-Ottawa Scale score, 4-6), and 15 were low quality (Newcastle-Ottawa Scale score, <4) ( Appendix 4 ). The agreement between the reviewers was almost perfect for study selection, data extraction, and risk of bias assessment, with kappa values 0.975, 0.942, and 0.967, respectively (with respective asymptomatic standard errors of 0.004, 0.015, and 0.033).
Assessment of publication bias
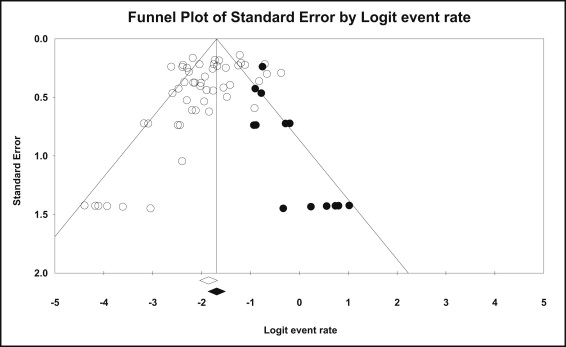
Inspection of the funnel plot hinted that there might be publication bias. In addition, both the tests of Begg and Mazumdar ( P = 0.03) and Egger et al ( P = 0.006) showed evidence of publication bias, and the trim-and-fill analysis, after taking into consideration possible missing studies ( Fig 2 ), yielded an adjusted overall failure rate of 15.6% (95% CI, 13.3-18.1) ( Appendix 5 ).
Only 1 risk factor analysis, that of the jaw of miniscrew implant insertion, included an adequate number of studies (>10) for the assessment of publication bias. After inspection of an asymmetric funnel plot, the test of Begg and Mazumdar gave a nonsignificant result, P = 0.108, and the test of Egger et al gave a significant result, P = 0.085. The trim-and-fill analysis indicated an initial underestimation of effects, yielding an increased adjusted relative risk of 2.10 (95% CI, 1.49-2.96).
Overall miniscrew implant failures
In the 52 studies included in the analysis, a total of 4987 miniscrew implants were placed in 2281 patients to reinforce orthodontic anchorage ( Appendix 6 ). The reported percentages of failures ranged between 0.0% and 40.8%. Meta-analysis of all trials with the fixed-effect model resulted in significant heterogeneity among the studies ( P <0.001; I 2 = 74%) ( Appendix 5 ).
By using the random-effects model, the meta-analysis of all trials yielded an overall failure rate of 13.5% (95% CI, 11.5-15.8) ( Fig 3 ), and the meta-analysis of trials with 100 or more miniscrew implants (including 3385 implants) yielded a summary failure estimate of 14.0% (95% CI, 11.5-17.0) ( Appendix 5 ).
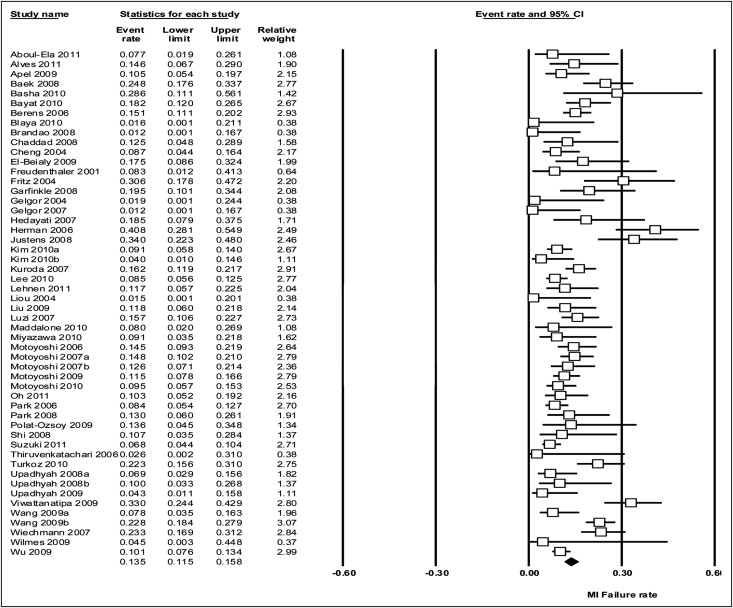
Risk factors associated with failure of miniscrew implants
A total of 30 of the 52 studies, including 4008 miniscrew implants placed in 1827 patients, reported 2 or more failure rates in relation to various factors. The remaining 22 studies did not provide compatible data for the investigation of risk factors, and thus they were used only for the overall estimate of failures. The miniscrew implant failure rates according to the various factors are presented in Table III . The factors investigated were divided into categories regarding patient, clinician, miniscrew implant, insertion procedure, miniscrew implant use during orthodontic treatment, and treatment complication characteristics.
| Factor | Groups | Number of studies | Heterogeneity ( P value) | Event rate (%) | 95% CI | P value (between SGs) | |
|---|---|---|---|---|---|---|---|
| Patient-related factors | Sex | Male | 9 | 0.006 | 13.4 | 8.4-20.7 | 0.907 |
| Female | 9 | <0.001 | 12.9 | 8.9-18.5 | |||
| Smoking | Nonsmokers | 1 | 1.000 | 9.6 | 4.6-18.8 | 0.002 ∗∗ | |
| Smokers | 1 | 1.000 | 35.1 | 21.6-51.5 | |||
| Smokers SG | Light (<10/day) | 1 | 1.000 | 11.1 | 2.8-35.2 | 0.007 ∗∗ | |
| Heavy (>10/day) | 1 | 1.000 | 57.9 | 35.6-77.4 | |||
| Age (y) | Adult (>20) | 5 | 0.136 | 15.5 | 11.2-21.0 | 0.575 | |
| Adolescent (<20) | 5 | <0.001 | 12.6 | 6.4-23.3 | |||
| Malocclusion | Class I | 2 | <0.001 | 23.4 | 4.8-65.1 | 0.191 | |
| Class II | 2 | 0.034 | 17.3 | 5.1-45.1 | |||
| Class III | 1 | 1.000 | 2.9 | 0.4-18.1 | |||
| Skeletal sagittal: ANB (°) | <0 | 1 | 1.000 | 11.8 | 5.4-23.8 | 0.002 ∗∗ | |
| 0-4 | 1 | 1.000 | 52.2 | 32.5-71.2 | |||
| >4 | 1 | 1.000 | 25.7 | 14.0-42.5 | |||
| Skeletal vertical: FMA (°) | Low (20) | 2 | 0.117 | 16.6 | 8.9-28.3 | 0.836 | |
| Middle (30) | 2 | 0.016 | 18.3 | 7.2-39.1 | |||
| High (40) | 2 | 0.032 | 9.3 | 1.0-51.0 | |||
| Skeletal vertical: Sn-GoGn (°) | Low (28) | 1 | 1.000 | 10.0 | 3.3-26.8 | 0.456 | |
| Middle (38) | 1 | 1.000 | 10.1 | 6.1-16.4 | |||
| High (48) | 1 | 1.000 | 2.9 | 0.4-18.1 | |||
| PI (%) | <20 | 1 | 1.000 | 37.9 | 22.4-56.4 | 0.187 | |
| 20-40 | 1 | 1.000 | 44.4 | 17.7-74.9 | |||
| >40 | 1 | 1.000 | 8.3 | 1.2-41.3 | |||
| GI (%) | <20 | 1 | 1.000 | 36.4 | 23.6-51.4 | 0.037 ∗ | |
| 20-40 | 1 | 1.000 | 92.9 | 42.3-99.6 | |||
| Oral hygiene | Good | 2 | 0.872 | 7.5 | 5.0-11.1 | 0.376 | |
| Bad | 2 | 0.979 | 9.8 | 6.3-14.8 | |||
| Clinician-related factors | Number of clinicians | 1 | 1 | 1.000 | 8.6 | 5.3-13.8 | 0.896 |
| 2 | 1 | 1.000 | 8.1 | 3.9-16.1 | |||
| Clinician | Professor | 1 | 1.000 | 1.9 | 0.3-12.0 | 0.005 ∗∗ | |
| PG student | 1 | 1.000 | 29.2 | 14.6-49.8 | |||
| Learning curve (per 18 MIs) | 1st | 1 | 1.000 | 25.0 | 13.6-41.5 | 0.009 ∗∗ | |
| 2nd | 1 | 1.000 | 8.8 | 4.0-18.3 | |||
| 3rd | 1 | 1.000 | 2.1 | 0.3-13.6 | |||
| 4th | 1 | 1.000 | 4.3 | 1.1-15.8 | |||
| Miniscrew-related factors | Product | AbsoAnchor | 3 | <0.001 | 16.8 | 6.4-37.4 | 0.223 |
| Aarhus | 1 | 1.000 | 13.0 | 6.3-24.8 | |||
| Osteomed | 1 | 1.000 | 15.8 | 5.2-39.2 | |||
| LOMAS | 2 | 0.416 | 5.9 | 3.3-10.3 | |||
| A1 | 1 | 1.000 | 20.0 | 7.7-42.8 | |||
| Microsrew; Mondeal | 1 | 1.000 | 5.3 | 0.7-29.4 | |||
| C-Implant | 1 | 1.000 | 8.5 | 5.6-12.5 | |||
| KLS-Martin | 1 | 1.000 | 20.0 | 2.7-69.1 | |||
| Diameter (mm) | 1.1 | 1 | Meta-regression | 0.387 | |||
| 1.2 | 3 | ||||||
| 1.3 | 2 | ||||||
| 1.4 | 3 | ||||||
| 1.5 | 2 | ||||||
| 1.6 | 1 | ||||||
| 1.7 | 1 | ||||||
| 1.8 | 1 | ||||||
| 2 | 6 | ||||||
| Diameter category (mm) | 1.1-1.3 | 4 | 0.042 | 10.9 | 7.7-15.3 | 0.729 | |
| 1.4-1.6 | 5 | 0.796 | 12.7 | 8.1-19.3 | |||
| 1.7+ | 6 | 0.013 | 14.3 | 7.4-25.8 | |||
| Thread length (mm) | 6 | 3 | Meta-regression | 0.183 | |||
| 7 | 2 | ||||||
| 8 | 6 | ||||||
| 8.5 | 1 | ||||||
| 9 | 1 | ||||||
| 10 | 3 | ||||||
| 11 | 2 | ||||||
| 12 | 2 | ||||||
| 13 | 1 | ||||||
| 14 | 1 | ||||||
| 15 | 1 | ||||||
| Thread length category (mm) | 5-8 | 11 | 0.101 | 12.3 | 8.3-17.9 | 0.281 | |
| 8.5-12 | 9 | <0.001 | 20.1 | 10.8-34.3 | |||
| 13-15 | 3 | 0.561 | 7.8 | 1.9-26.7 | |||
| Head length (mm) | 4.5 | 1 | 1.000 | 8.3 | 1.2-41.3 | 0.806 | |
| 2.5 | 1 | 1.000 | 12.5 | 0.7-73.4 | |||
| Thread design | Self-drilling | 3 | 0.824 | 7.7 | 4.8-12.0 | 0.210 | |
| Not self-drilling | 3 | <0.001 | 17.3 | 5.1-44.9 | |||
| Thread surface | Machined | 1 | 1.000 | 17.6 | 5.8-42.7 | 0.366 | |
| Sandblasted & acid-etched | 1 | 1.000 | 6.7 | 0.9-35.2 | |||
| Insertion-related factors | Cortical notching | No | 3 | 0.734 | 6.8 | 4.1-11.1 | 0.154 |
| Yes | 3 | <0.001 | 13.7 | 5.9-28.4 | |||
| Flap | No | 1 | 1.000 | 51.3 | 36.0-66.4 | 0.037 ∗ | |
| Yes | 1 | 1.000 | 4.5 | 0.3-44.8 | |||
| Insertion torque (Ncm) | <10 | 2 | 0.925 | 8.8 | 5.3-14.2 | 0.004 ∗∗ | |
| >10 | 2 | 0.172 | 29.9 | 15.5-49.7 | |||
| Insertion angle (°) | 10-20 | 1 | 1.000 | 9.0 | 4.1-18.5 | 0.113 | |
| 30-40 | 1 | 1.000 | 4.8 | 2.0-10.9 | |||
| 90 | 1 | 1.000 | 14.8 | 7.6-26.9 | |||
| Screw head exposed | No | 2 | <0.001 | 21.2 | 1.3-84.8 | 0.696 | |
| Yes | 2 | 0.849 | 12.8 | 8.3-19.1 | |||
| Cortical bone thickness (mm) | ≥1 | 2 | 0.892 | 8.3 | 5.3-12.8 | 0.003 ∗∗ | |
| <1 | 2 | 0.592 | 21.3 | 13.7-31.7 | |||
| Jaw | Maxilla | 17 | 0.014 | 12.0 | 9.6-14.9 | 0.012 ∗ | |
| Mandible | 17 | <0.001 | 19.3 | 14.3-25.6 | |||
| Side | Left | 8 | <0.001 | 13.2 | 7.9-21.3 | 0.382 | |
| Right | 8 | <0.001 | 17.4 | 11.9-24.6 | |||
| Region | Posterior | 2 | <0.001 | 16.1 | 4.7-42.6 | 0.771 | |
| Anterior | 2 | 0.680 | 22.0 | 2.9-72.6 | |||
| Soft tissue | Keratinized | 3 | 0.459 | 12.5 | 7.0-21.5 | 0.450 | |
| Nonkeratinized | 3 | <0.001 | 21.6 | 5.5-56.7 | |||
| Site | Interradicular | 5 | 0.268 | 10.9 | 8.3-14.0 | 0.412 | |
| Palate | 5 | 0.108 | 15.6 | 6.6-32.7 | |||
| Palate SG | Midpalatal | 4 | 0.113 | 16.8 | 6.6-36.7 | 0.135 | |
| Parapalatal | 1 | 1.000 | 7.5 | 4.4-12.5 | |||
| Interradicular SG | Teeth: P2M1 | 1 | 1.000 | 23.3 | 15.0-34.3 | 0.553 | |
| Teeth: M1M2 | 1 | 1.000 | 28.6 | 16.1-45.4 | |||
| Root contact | Yes | 4 | 0.102 | 29.9 | 21.0-40.7 | <0.001 ∗∗∗ | |
| No | 4 | 0.122 | 7.8 | 3.9-15.0 | |||
| Treatment-related factors | Two MIs splinted | Yes | 1 | 1.000 | 4.1 | 1.7-9.4 | 0.003 ∗∗ |
| No | 1 | 1.000 | 17.6 | 10.5-27.9 | |||
| Loading time | Up to 2 weeks | 3 | 0.125 | 26.8 | 16.4-40.6 | 0.304 | |
| After 2 weeks | 3 | 0.073 | 15.6 | 5.6-36.7 | |||
| Tooth movement | Intrusion | 1 | 1.000 | 11.1 | 4.2-26.1 | 0.826 | |
| Distalization | 1 | 1.000 | 7.3 | 2.8-17.8 | |||
| Mesialization | 1 | 1.000 | 10.0 | 1.4-46.7 | |||
| En-masse retraction | 1 | 1.000 | 11.3 | 5.7-20.9 | |||
| Combination | 1 | 1.000 | 4.0 | 0.6-23.5 | |||
| Treatment duration (months) | <6 | 1 | 1.000 | 27.3 | 9.0-58.6 | 0.046 ∗ | |
| >6 | 1 | 1.000 | 8.1 | 4.9-12.9 | |||
| Complication-related factors | Inflammation | Yes | 2 | <0.001 | 48.7 | 3.4-96.2 | 0.260 |
| No | 2 | 0.001 | 10.3 | 2.2-36.9 | |||
| Mobility | Yes | 1 | 1.000 | 24.4 | 14.1-39.0 | <0.001 ∗∗∗ | |
| No | 1 | 1.000 | 1.4 | 0.4-5.6 | |||
| Unclear | 1 | 1.000 | 14.0 | 6.4-27.8 | |||
| MI reinstallation | No | 1 | 1.000 | 24.8 | 17.6-33.7 | 0.130 | |
| Yes | 1 | 1.000 | 38.2 | 23.7-55.3 | |||
| MI reinstallation site | Same | 1 | 1.000 | 31.6 | 14.9-54.8 | 0.371 | |
| Adjacent | 1 | 1.000 | 46.7 | 24.1-70.7 | |||
| Failure type | Complete | 2 | <0.001 | 18.0 | 4.7-49.4 | 0.719 | |
| Complete and partial | 2 | 0.007 | 23.3 | 10.1-45.2 |
∗ Statistically significant at P <0.05.
No difference in the miniscrew implant failure rates was observed for the following factors: patient sex (9 studies) and patient age (with age 20 years for the dichotomization; 5 studies).
The small number of included studies precluded a safe assessment of clinician-related factors.
The miniscrew implant’s thread diameter and thread length (tested through subgroup analysis; mean studies per group, 5 studies and 7 studies, respectively) were found not to be associated with the miniscrew implant failure rates.
No significant differences of the miniscrew implant failure rates were observed with regard to side of placement (left vs right; 8 studies) and site of placement (interradicular vs palatal; 5 studies). In addition, higher overal failure rates were observed when the miniscrew implants were inserted in the mandible than in the maxilla (19.3% and 12.0%, respectively; 17 studies; P = 0.012) (relative risk = 1.56; 95% CI, 1.13-2.15; P = 0.007). These reported overall failure rates were further stratified by jaw of insertion: separately for miniscrew implants placed in the maxilla and in the mandible ( Table IV ), but the number of included studies per subgroup was low.
| Jaw | Factor | Group | Studies | Heterogeneity ( P value) | Event rate (%) | 95% CI | P value (between SGs) |
|---|---|---|---|---|---|---|---|
| Maxilla | Side | Left | 4 | 0.035 | 15.8 | 7.4-30.7 | 0.307 |
| Right | 4 | 0.035 | 20.1 | 12.6-30.4 | |||
| Region | Posterior | 2 | 0.459 | 23.7 | 9.7-47.4 | 0.006 ∗∗ | |
| Anterior | 1 | 1.000 | 4.2 | 1.7-9.6 | |||
| Soft tissue | Keratinized | 1 | 1.000 | 8.8 | 2.9-24.0 | 0.635 | |
| Nonkeratinized | 1 | 1.000 | 6.4 | 2.9-13.5 | |||
| Cortical bone thickness (mm) | ≥1 | 1 | 1.000 | 3.0 | 0.4-18.6 | 0.031 ∗ | |
| <1 | 1 | 1.000 | 26.1 | 12.2-47.2 | |||
| Site 1 | Alveolar process | 4 | 0.069 | 12.0 | 7.0-19.9 | 0.924 | |
| Palate | 4 | 0.027 | 11.1 | 2.3-39.8 | |||
| Site 2 | Midpalatal | 2 | 0.017 | 6.6 | 0.2-73.3 | 0.948 | |
| Interradicular | 2 | 0.538 | 7.4 | 4.5-11.8 | |||
| Interradicular SG 1 | Buccal | 2 | 0.802 | 9.7 | 4.4-20.0 | 0.107 | |
| Palatal | 2 | 0.381 | 21.1 | 11.5-35.4 | |||
| Interradicular SG 2 | Teeth: P1P2 | 1 | 1.000 | 6.3 | 0.4-53.9 | 0.357 | |
| Teeth: P2M1 | 2 | 0.187 | 18.7 | 9.3-34.0 | |||
| Teeth: M1M2 | 1 | 1.000 | 28.6 | 16.1-45.4 | |||
| Mandible | Side | Left | 3 | 0.516 | 15.9 | 9.0-26.5 | 0.935 |
| Right | 3 | 0.899 | 15.4 | 8.7-25.7 | |||
| Region | Posterior | 3 | <0.001 | 18.8 | 7.4-40.3 | 0.679 | |
| Anterior | 3 | 0.837 | 23.7 | 11.4-42.9 | |||
| Site 1 | Symphysis | 1 | 1.000 | 23.5 | 9.1-48.6 | 0.271 | |
| Retromolar | 1 | 1.000 | 20.0 | 5.0-54.1 | |||
| Interradicular | 1 | 1.000 | 9.7 | 4.7-19.0 | |||
| Interradicular SG 1 | Buccal | 2 | 0.905 | 9.1 | 3.0-24.7 | <0.001 ∗∗∗ | |
| Lingual | 1 | 1.000 | 73.3 | 46.7-89.6 | |||
| Interradicular SG 2 | Teeth: P1P2 | 1 | 1.000 | 5.6 | 0.3-50.5 | 0.565 | |
| Teeth: P2M1 | 1 | 1.000 | 16.7 | 1.0-80.6 |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


