Figure 4.1A • Scanning electronmicrograph (SEM) of the root canal dentine conventionally treated (A), Er:YAG laser (B) and Nd:YAG laser (C). Original magnifications: 50X (A, D); 1000 X (B, E) and 2000X (C, F).
Until now the studies agree that the use of the Nd:YAG laser does not reduce the mean amounts of debris and smear layer compared to a non irradiated root canal conventionally treated (Barbakow et al., 1999; Kaitsas et al., 2001).
In a review, Stabholz et al., 2002 tried to explain these results by emphasizing the possible limitations that may be associated with use of lasers in the root canal system.
They suggested that it is almost impossible to obtain uniform coverage of the canal surface using a laser because the emission of laser’s energy from the tip of the optical fiber of the laser guide is directed along the root canal and not necessary laterally to the root canal walls.
Altamura et al. (2003) observation corroborate the Stabholz statement who showed that complete cleaning of root canal walls with Nd:YAG and Er:YAG laser radiation is still difficult and stated that is still a need to develop devices to irradiate the entire root canal walls.
In 1997, the Er:YAG laser received the FDA approval for using in hard tissues (Cozean et al., 1997).
One year later, the same laser device was successfully applied in Endodontics in order to remove debris and smear layer produced during root canal preparation.
Then, it was demonstrated that Er:YAG laser is effective in removing debris and smear layer from root canal walls (Matsuoka et al., 1998; Takeda et al., 1998).
Until recently, the main field of Er:YAG laser application was the removal of dental hard substances for cavity preparation.
Nowadays, several new delivery systems are available, allowing the application of the Er:YAG laser in Endodontics.
Through the development of special fibers for the wavelength around the 3000nm, the use of the Er:YAG laser in the root canal has become feasible.
The typical morphology of the root canal walls treated by Er:YAG laser is characterized by clean areas, always free of smear layer showing open dentinal tubules in a globular surface (Figure 4.1B).
Er:YAG laser was the most effective for removing the smear layer from the root canal wall when compared to the Argon (Takeda comparing 3 types), the Nd:YAG (Takeda comparing 3 types, Ramalho et al., 2005) and to CO2 lasers (Takeda). Moreover, the Er:YAG laser irradiation with special microprobes (200-400 μm diameter and 20 mm in length) are effective in shaping, cleaning, and enlarging straight root canals faster and more efficiently than conventional methods (Kessler et al., 2002).
Additionally, the use of the Er:YAG laser combined with rotatory and manual techniques improves the cleanliness of root canals (Biedma et al., 2005).
Thermal effect on periodontal tissues during root canal preparation using the Er: YAG laser is minimal (Kimura et al., 2002 and 2004; Schoop et al., 2002). Thus, the potential thermal damage of periodontal tissues is not a concern when using Er:YAG laser in the root canal (Stabholz et al., 2004).
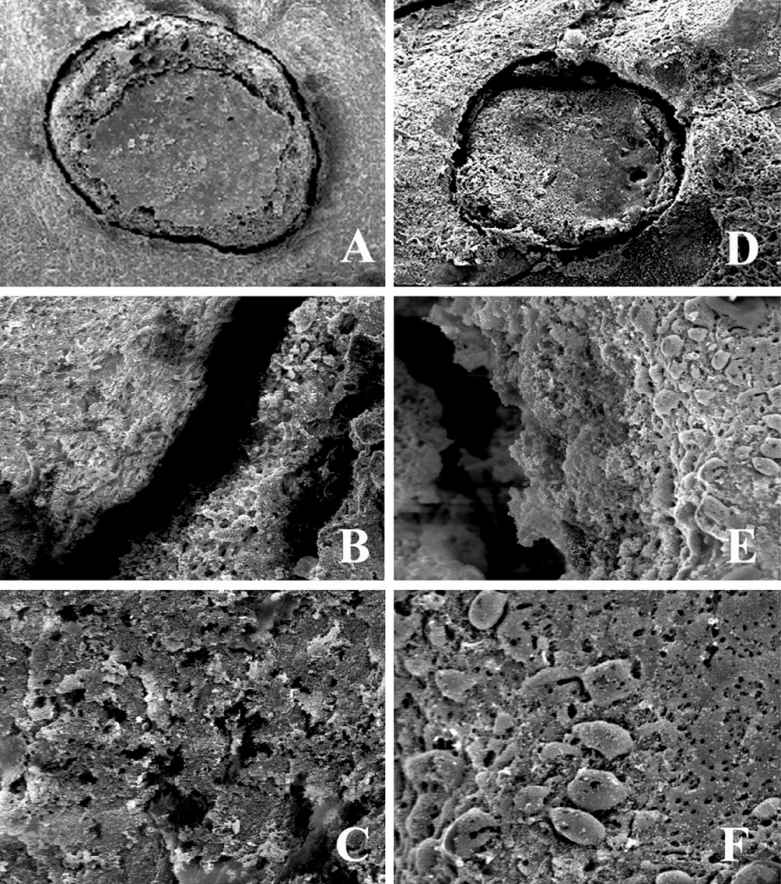
Figure 4.1B • Electronmicrographs of dental root end cut surfaces obtained with Er:YAG laser (A-C) and Er:YAG laser (D-F) followed by Nd:YAG laser. Original magnification: 500x.
II. Bacterial reduction
The first studies on sterilization effect of lasers in Endodontics were carried out by Hooks et al., 1980 using the CO2 laser. This laser was successfully applied in endodontic sterilization. In 1994, Rooney et al. investigated the effect of pulsed Nd:YAG laser irradiation on bacteria in a laboratory model. This model simulated the number of organisms that could be expected to occur in an infected root canal. Bacterial reduction was achieved indicating a potential role of the Nd:YAG laser in Endodontics. When compared with the conventional root canal treatment using NaOCl, the Nd:YAG laser irradiation showed similar results in bacterial reduction; however, complete root canal sterilization was not obtained (Hardee et al., 1994; Fegan & Steiman,1995). On the other hand, in 1995 Moshonov et al. showed that the Nd:YAG presented a less effective antibacterial action, once the laser irradiation was able to significantly reduced the number of bacteria in the root canal while NaOCl irrigation effectively disinfected the canals.
In vitro studies showed that Nd:YAG laser has antibacterial effect in infected root canal against several bacteria such as: Bacillus stearothermophilus spores (Hardee et al., 1994; Fegan et al., 1995), Enterococcus faecalis (Moshonov et al., 1995; Gutknecht et al., 1996), Streptococcus feacalis, Escherichia coli (Gutknecht et al., 1997).
Following an in vitro investigation, Moritz et al. in 1997 introduced the diode laser system emitting in the near-infrared ranged to root canal treatment. A follow up examination in vivo showed that irradiate root canals with 810 nm diode laser could achieve a significant elimination of Streptococcus and Staphilococcus (Moritz et al., 1997).
Gutknecht et al., determined the microbial effect of the Ho:YAG laser system in an in vitro study, revealing highly favorable results. 99.98% of the Enteroccocus injected in the root canals could be eliminated at setting of 2W and 5Hz.
Later on in vitro studies using Er:YAG laser indicated a significant bacterial reduction in the root canal (Moritz et al., 1999). Additionally, Moritz et al. compared the antibacterial effectiveness of Er:YAG, Ho:YAG, and Nd:YAG lasers systems and their findings indicated that the best results were obtained by Er:YAG laser, followed by Nd:YAG and Ho:YAG laser, all at output of 1.5W.
New studies were carried out under in vitro conditions analyzing bactericidal effects of laser radiation after penetration of root canal dentin of different thickness. The Nd:YAG laser irradiation, although weakened by penetrating dentin layers, has bactericidal effects also in depths of 1,000 μm and above (Klinkle et al., 1997; Moritz et al., 2000). According to Vaarkamp et al. (1995) one possible explanation is the ability of the enamel prisms and dentin tubules to act as optical fibers, thus propagating laser light to the dentinal periphery of the root.
Moreover, despite the antimicrobial effect of lasers in dentin walls of root canal, the elimination of microorganims may not be uniform because of the different susceptibility of the species involved taking into account the specific architecture of Gram negative and Gram positive microorganisms. The construction of the cell wall of Gram positive is crucial for their individual sensitivity to laser treatment (Moritz et al., 2000).
III. Endodontic surgery
The failures of the apical surgeries have been related to the egress of microorganism, their products and organic fluids from the root canal system into the periapical tissues. It is assumed that the irritants penetrate mainly through a gap present between the retrograde filling and dentin or through the dentin of the cut surface after apicectomy (Altonem et al., 1976; Kaplan et al., 1982; Stabholz et al., 1985; Molven et al., 1991; Friedman et al., 1991; Stabholz et al., 2004).
For these reasons, several techniques, methods and new materials have been developed to enhance the apical surgery in order to increase the sealing ability of the retrograde filling material (Lage- Marques et al., 1997; Eduardo et al., 2001).
Same laser wavelengths are able to remove the dental hard tissue by ablation process. Then, they can be used for cavity preparation and also for apicectomy. In vitro studies of apicectomies using the laser irradiation have been conducted to obtain a smoother and less permeable cut dentin surface producing the melting and recristallization of the dentinal structure with the closure of the dentinal tubules (Melcer et al., 1981; Miserendino et al., 1988; Moritz et al., 1997; Gouw-Soares et al., 1996; 1999; 2001; 2004; Stabholz et al., 1992; Oliveira et al., 2004).
The CO2 laser was the first one to be used experimentally for the ablation of dental hard tissue by Stern and Sognnaes. However, nowadays, it is well known that continuous wave does not remove dental tissue properly as the temperature rise causes thermal damage to surrounding tissue. For this reason the 10.6μm CO2 laser emitted in a continuous wave should be restricted to soft tissue use (Eduardo et al., 2001). However recent studies have demonstrated that the use of a 9.6μm CO2 laser with pulsed emission to irradiate dentine and enamel samples in cavity preparations and apicoectomies does not cause carbonization of the hard tissues (Fried et al., 1994; 1997; 1999; Wigdor et al., 1997; Gouw-Soares et al., 2000; 2004; Moshonov et al., 2000; Stabholz et al., 2000). The improved results seem to be obtained when the tissue is cooled with water spray during the irradiation, as with other laser systems with the purpose of dental hard tissue ablation without thermal damages (Eduardo, 2001).
Additionally, previous studies demonstrated that the use of wavelengths between 9.3 and 10.6 μm with pulsed emission when irradiating dentin and enamel samples in cavity preparations and apicectomies, have not caused the carbonization of the hard tissues (Wigdor et al., 2000; Stabholz et al., 2000).
In vitro studies were developed using the pulsed Er:YAG laser to cut the apical root in apicoectomy (Gouw Soares et al., 1996; Komori et al., 1997; Paghdiwala et al., 1993). All of them have demonstrated by SEM examination that the resection surface presented a slightly irregular clean surface, no smear layer, with dentinal tubules exposed with small thermal damage. Those aspects evidenced by electron-micrographies were similar to the ones found in vitro studies of the Er:YAG laser irradiation on dentine surface in cavity preparations as well as on root canal dentin (Hibst et al., 1989; Keller et al., 1989; Tanji et al.,1998; Tanji et al., 1999).
On the other hand, the in vitro studies using Nd:YAG have shown a reduction in the penetration of dye or bacteria through resected roots. It was suggested that the reduced permeability in the lased specimens probably was resultant of structural changes in the dentin following laser application. Although SEM examinations showed melting, solidification and recrystallization of the hard tissue, the structural changes were not uniform (Stabholz et al., 1992; Arens et al., 1993; Wong et al., 1994). According to STABHOLZ et al. (2004), it was postulated that this is the reason why the permeability of the dentin was reduced but not completely prevented.
Gouw-Soares proposed the use of an association of the Er:YAG and Nd:YAG lasers in apicoectomy in a attempt to obtain a less permeable apical surface since previous studies with 1064nm Nd:YAG laser demonstrated its capacity to promote vitrified dentinal areas by fusion, dentin resolidification and parcial sealing of the dentinal tubules with a decrease of the surface permeability without thermal damage (Gouw Soares et al., 1999). Then, the same protocol was used in clinical procedures of apicectomy in conjunction with Nd:YAG and the As- Ga- Al low intensity laser. Three years later a follow up examination of the same clinical case showed a radiographically significant decrease of the radiolucent periapical area and no clinical signs and symptoms, indicating that the use of those lasers could be considered a suitable and useful alternative method to perform an apicoectomy (Gouw Soares et al., 2001).
IV. Photo ActivATed Disinfection (PAD)
Recently a novel method of disinfection for using in Endodontics has become available. As a new approach, could be an alternative to conventional therapeutic methods. PAD is based on the principle that a photoactivatable substance, the photosensitizer, binds to the target cell and can be activated by light of a suitable wavelength. During this process, free radicals are formed (among them singlet oxygen), which then produce an effect that is toxic for the bacteria. To have a specific toxic effect on bacterial cells, the respective photosensitizer needs to have selectivity for prokaryotic cells. PAD is an alternative approach to microbial killing in the root canal system by laser light and involves the use of low-power lasers to drive a photochemical reaction that produces reactive oxygen species. For example, Tolonium Chloride, an exogenous photosensitizer, is able to kill all types of bacteria. In vitro studies of PAD have demonstrated its ability to kill photosensitised oral bacteria, such as E. faecalis (Silva et al., 2006), and more recently microbial killing, such as Fusobacterium Nucleatum, Prevotella intermedia, Streptoccocus intermedius and Peptostreptoccocus micros and Actinomicetocomitans (Prates et al., 2006), in vivo in the root canal system has been demonstrated (Bonsor et al., 2006). While PAD can be undertaken as part of the routine disinfection of the root canal system, it has also the potential use to eradicate persistent endodontic infections for which conventional methods have been unsuccessful (Lee et al., 2004; Bonsor et al., 2006).
V. Phototherapy
The use of laser therapy as an agent reinforcing conventional treatment of the endodontics is more and more common. In the physiotherapy of Endodontics the biostimulatory laser is eagerly used because of its action which accelerates the wound healing and also because of its antiedematous, anti-inflammatory and analgesic action. In combination with the conventional treatment the laser stimulation shortens its duration and leads the elimination of pain faster than usual. In fact, the biostimulatory effect of low power lasers can be proved in vitro using cell culture. Using mesenchymal cells in culture, such as fibroblasts and osteoblasts, our research group has shown that lasers at low energy are able to improve cell adhesion and cell growth (Pereira et al., 2002; Almeida-Lopes et al., 2001; Marques et al., 2004; Fujihara et al., 2006; Azevedo et al.; 2006). However, the photostimulation in vitro in the condition tested was not able to increase protein cell secretion (Pereira et al., 2002; Marques et al., 2004; Fujihara et al., 2005). Diode lasers with different wavelengths were used. For Almeida-Lopes et al (2001) the infrared laser (780 nm) induced significantly higher cell growth than the visible laser (670 nm). However, with others wavelengths there were no differences in the proliferative effect between red (693 nm) and infrared (786 nm). The irradiation time was found an important factor in the fibroblasts biostimulation (Almeida-Lopes et al., 2001). Later on, Pereira et al. (2002) showed that a diode laser (904 nm) stimulated the cell growth within the parameters of 2 to 4 J /cm2, and that effect was lost when 5 J/cm2 was used. These authors also showed no changes at the procollagen content of irradiated fibroblasts. Investigating the collagen secretion of irradiated fibroblasts, Marques et al., (2004) showed that the type I collagen as well as the total protein secreted by irradiated cells were significantly reduced in irradiated fibroblasts. They suggested that this result could be explained based on the ultrastructural changes observed in the cells. However, there are controversies on the effect of laser irradiation on collagen metabolism in vitro. Studies of Yamamoto et al., (1996) and Skinner et al. have suggested that the laser would increase collagen synthesis. The different effects can reside on differences in laser irradiation parameters used.
Working with other cell type, Fujihara et al. (2005) showed that the low intensity laser therapy stimulated the proliferation of osteoblast-like cells even under the influence of dexamethasone. This study suggested that phototherapy can be of importance as co adjuvant in bone clinical manipulation in order to accelerate bone regeneration. Then could be important in periapical surgeries.
The in vitro studies in biostimulation by low power laser are concerned with wavelengths and energy densities, however, we have shown that, with the same energy density (3J/cm2) but changing the power densities, the effect on fibroblasts growth was modified. In fact, this study showed that the power density influences cell growth in an inversely proportional manner (Azevedo et al., 2006).
In vivo studies are also available showing the antiinflamatory effect of phototherapy (Honmura et al., 1992 and 1993; Giuliani et al., 2004; Albertini et al., 2004; Lopes-Martins et al., 2005; Ambieri et al., 2006; Meneguzo et al., 2006). However new studies and clinical trials must be done in order to validate the laser technology in Endodontics.
REFERENCES
1. Lage-Marques JL, Eduardo C, Matsumoto K. A study on morphological changes of the root canal walls lased by pulsed Nd:YAG laser. J Japan Endodontic Assoc 1995; 16 (1): 64-69.
2. Lage-Marques JL, Eduardo CP. O uso do laser em Endodontia. São Paulo, Pancast, 1998; 339-414.
3. Moritz A, Jakolitsch S, Goharkhay K, Schoop U, Kluger W, Mallinger R, Sperr W, Georgopoulos A. Morphologic changes correlating to different sensitivities of Escherichia coli and enterococcus faecalis to Nd:YAG laser irradiation through dentin. Lasers Surg Med 2000; 26(3):250-61.
4. Eduardo CP, Gouw-Soares S. The use of lasers in endodontics. J Oral Appl 2001; 1:221-226.
5. Eduardo CP, Gouw-Soares S. The use of lasers for endodontics applications in dentistry. Med Laser Appl 2001; 16: 231- 243.
6. Stabholz A, Moshonov J. Incorporating laser technology into endodontic treatment. Alpha Omegan 2004 Dec; 97(4):75-81.
7. Stabholz A, Sahar-Helft S, Moshonov J. Lasers in endodontics. Dent Clin North Am 2004 Oct; 48(4):809-32. Review.
8. Gutknecht N, Franzen R, Schippers M, Lampert F. Bactericidal effect of a 980-nm diode laser in the root canal wall dentin of bovine teeth. J Clin Laser Med Surg 2004 Feb; 22(1):9-13.
9. Adrian JC. Pulp effects of neodymium laser. A preliminary report. Oral Surg Oral Med Oral Pathol 1977 Aug; 44(2):301-5.
10. McComb D, Smith DC. A preliminary scanning electron microscope study of root canal instrumentation in endodontic therapy. J Endod 1975; 1: 238 -42.
11. Moodnik RM, Dorn SO, Feldman MJ, Levey M, Borden BG. Efficacy of biomechanical instrumentation: a scanning electron microscopy study. J Endod 1976; 2: 261-6.
12. Mader CL, Baumgartner JC, Peters D. Scanning electron microscopic investigation of the smeared layer on root canal walls. J Endod 1984; 10:477-83.
13. Matsumoto K. Lasers in endodontics. Dent Clin N Am 2000; 44:889-906.
14. Kimura Y, Yonaga K, Yokoyama K, Kinoshita J, Ogata Y, Matsumoto K. Root surface temperature increase during Er:YAG laser irradiation of root canals. J Endod 2002 Feb; 28(2):76-8.
15. Kimura Y, Yonaga K, Murakoshi M, Yokoyama K, Watanabe H, Matsumoto K. Effects on periradicular periodontal tissues of root canal irradiation with Er:YAG laser in rats. Photomed Laser Surg 2004 Aug; 22(4):335-41.
16. Biedma BM, Varela Patino P, Park SA, Barciela Castro N, Magan Munoz F, Gonzalez Bahillo JD, Cantatore G. Comparative study of root canals instrumented manually and mechanically, with and without Er:YAG laser. Photomed Laser Surg 2005 Oct; 23(5):465-9.
17. Harashima T, Takeda FH, Kimura Y, Matsumoto K. Related Articles, Links Effect of Nd:YAG laser irradiation for removal of intracanal debris and smear layer in extracted human teeth. J Clin Laser Med Surg 1997; 15(3):131-5.
18. Barbakow F, Peters O, Havranek L. Effects of Nd:YAG lasers on root canal walls: a light and scanning electron microscopic study. Quintessence Int 1999 Dec; 30(12):837-45.
19. Kaitsas V, Signore A, Fonzi L, Benedicenti S, Barone M.Effects of Nd: YAG laser irradiation on the root canal wall dentin of human teeth: a SEM study. Bull Group Int Rech Sci Stomatol Odontol 2001 Sep-Dec; 43(3):87-92.
20. Takeda FH, Harashima T, Kimura Y, Matsumoto K. Comparative study about the removal of smear layer by three types of laser devices. J Clin Laser Med Surg 1998 Apr; 16(2):117-22.
21. Takeda FH, Harashima T, Eto JN, Kimura Y, Matsumoto K. Effect of Er:YAG laser treatment on the root canal walls of human teeth: an SEM study. Endod Dent Traumatol 1998 Dec; 14(6):270-3.
22. Takeda FH, Harashima T, Eto JN, Kimura Y, Matsumoto K. Effect of Er:YAG laser treatment on the root canal walls of human teeth: an SEM study. Endod Dent Traumatol 1998 Dec; 14(6):270-3.
23. Takeda FH, Harashima T, Kimura Y, Matsumoto K. Efficacy of Er:YAG laser irradiation in removing debris and smear layer on root canal walls. J Endod 1998 Aug; 24(8):548-51.
24. Matsuoka E, Kimura Y, Matsumoto K. Studies on the removal of debris near the apical seats by Er:YAG laser and assessment with a fiberscope.J Clin Laser Med Surg 1998 Oct;16(5):255-61.
25. Gutknecht N, Behrens VG. Instrumentation of root canal walls with Nd-YAG laser ZWR. 1991 Oct; 100(10):748-50, 752, 755.
26. Goodis HE, White JM, Marshall SJ, Marshall GW Jr. Scanning electron microscopic examination of intracanal wall dentin: hand versus laser treatment. Scanning Microsc 1993 Sep; 7(3):979-87.
27. Gutknecht N, Behrens, VG. The Nd:YAG laser as an aid to root canal obturation. Milan, Monduzzi Editore, 79th Annual World Dental Congress of FDI, 1991; I 275-I 280.
28. Harashima T, Takeda FH, Kimura Y. Effect of Nd:YAG laser irradiation for removal of intracanal wall debris and smear layer in extracted human teeth. J Clin Laser Med Surg; 15:131-135.
29. Altamura C, Majori M,Bedini R, Filippini P. Evaluation of Nd:YAG laser effect on root canal walls. J Oral laser Appl 2003; 3(2):67-72.
30. Stabholz A, Sahar-Helft S, Moshonov J. Lasers in endodontics. Dent Clin North Am 2004 Oct; 48(4):809-32, vi. Review.
31. Cozean C, Arcoria CJ, Pelagalli J, Powell GL. Dentistry for the 21st century? Erbium:YAG laser for teeth. J Am Dent Assoc 1997 Aug; 128(8):1080-7.
32. Kesler G, Gal R, Kesler A, Koren R. Histological and scanning electron microscope examination of root canal after preparation with Er:YAG laser microprobe: a preliminary in vitro study. J Clin Laser Med Surg 2002 Oct; 20(5):269-77.
33. Schoop U, Moritz A, Kluger W, Patruta S, Goharkhay K, Sperr W, Wernisch J, Gattringer R, Mrass P, Georgopoulos A. The Er:YAG laser in endodontics: results of an in vitro study.Lasers Surg Med 2002;30(5):360-4.
34. Hooks TW, Adrian JC, Gross A, Bernier WE. Use of the carbon dioxide laser in sterilization of endodontic reamers. Oral Surg Oral Med Oral Pathol 1980 Mar; 49(3):263-5.
35. Rooney J, Midda M, Leeming J. A laboratory investigation of the bactericidal effect of an Nd:YAG laser. Br Dent J 1994 Jan 22; 176(2):61-4.
36. Hardee MW, Miserendino LJ, Kos W, Walia H. Evaluation of the antibacterial effects of intracanal Nd:YAG laser irradiation. J Endod 1994 Aug; 20(8):377-80.
37. Fegan SE, Steiman HR. Comparative evaluation of antibacterial effect of Nd: YAG laser irradiation. An in vitro study. J Endod 1995; 21:415-7.
38. Moshonov J, Orstavik D, Yamauchi S, Pettiette M, Trope M. Nd:YAG laser irradiation in root canal disinfection. Endod Dent Traumatol 1995 Oct; 11(5):220-4.
39. Gutknecht N, Nuebler-Moritz M, Burghardt SF, Lampert F. The efficiency of root canal disinfection using a holmium:yttrium-aluminum-garnet laser in vitro. J Clin Laser Med Surg 1997; 15(2):75-8.
40. Moritz A, Gutknecht N, Goharkhay K, Schoop U, Wernisch J, Sperr W. In vitro irradiation of infected root canals with a diode laser: results of microbiologic, infrared spectrometric, and stain penetration examinations. Quintessence Int 1997 Mar; 28(3):205-9.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


