In the human embryo, all organs—including the structures of the head and neck—develop from three germ layers: the ectoderm, mesoderm, and endoderm. These germ layers form during gastrulation, a differentiation process occurring at the beginning of week 3. (Note: All given time specifications refer to the gestational age of the embryo.) Once gastrulation has been completed, the formation of primordia, the earliest recognizable stages of organs, starts. By the end of week 8, organogenesis is completed, and during the remaining time of pregnancy (the fetal period) the organs continue to grow and mature.
Time Line of Head and Neck Development
In week 4, the brain develops at the cranial pole of the embryo by neurulation (discussed later). By the end of week 4, optic vesicles, which will substantially contribute to the formation of the eyes, have protruded from the developing brain. Together with the stomodeum, the primordium of the mouth, the optic vesicles are among the first discernible facial structures in the future head region. In week 3, five pharyngeal arches begin to form and subsequently, in weeks 5 and 6, further differentiate into important structures in the head and neck region. At the same time, mesenchymal tissue around the developing brain starts to form the skull. In weeks 7 and 8, the facial structures, such as jaw, nose, eyes, and ears, become more defined. During this late phase of organogenesis and in the following fetal period, the face takes on its characteristic human contours mostly by differential growth of its various structures. For example, early in organogenesis, the eyes are located at the lateral aspect of the developing head. During development, they appear to move closer to the midline because the portions of the face lateral to the eyes grow faster than the portion between the eyes.
Not only do the proportions of facial structures change during prenatal development, but the head-to-body ratio also changes. In the early fetus (week 12), the head constitutes about one third of the body length; this relation decreases to about one quarter of the body length at the time of birth and continues to decrease during postnatal development.
Neurulation and Formation of Neural Crest Cells as a Prerequisite for Head and Neck Development ( Figure 9-1 )
The formation of the head and neck strongly depends on the successful completion of neurulation (i.e., the formation of the central nervous system [CNS]). Early in week 4, parts of the most dorsal germ layer, the ectoderm, transform into the neural plate. The thickened epithelial cells, which form the neural plate, are located at and adjacent to the embryo’s craniocaudal axis. The neuronal plate invaginates along this axis, and the resulting neuronal folds separate from the ectoderm and fuse to form the neural tube, which in turn will develop into the CNS. The free edges of the remaining surface ectoderm fuse over the neural tube and thereby build a continuous layer, which later will differentiate into epidermis. In humans, the fusion of the neural folds starts at least at two discrete points along the craniocaudal axis and proceeds in cranial and caudal directions. The cranial portion of the neural tube, the future brain, closes completely on day 25. Failure of this fusion results in anencephaly and is incompatible with postnatal life. Anencephaly occurs with an incidence of approximately 1 in 4800 live births and is always accompanied by acrania, the partial or complete absence of the cranial vault ( Figure 9-2 ).
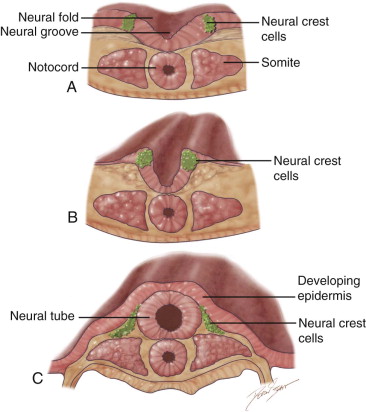
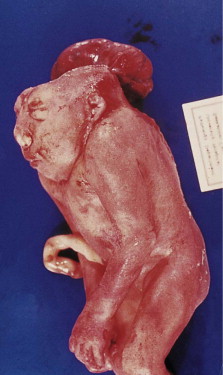
During the fusion of the neural folds into the neural tube, cells at the edge of the neural fold separate and form streaks of neural crest cells (NCCs) along the entire neural tube. NCCs undergo an epithelial-mesenchymal transition as they detach from the neural tube and migrate to various locations in the body. They form a vast array of structures, such as the ganglia of the autonomic nervous system, the enteric nervous system, and the adrenal medulla. In the head and neck region, NCCs give rise to much of the connective tissue in the craniofacial complex (skull, face, pharyngeal arches ). Therefore, neurocristopathies (i.e., the abnormal development of neural crest cells) often cause developmental defects that involve the craniofacial system (e.g., DiGeorge syndrome, Treacher Collins syndrome, and cleft formation).
Neurulation and Formation of Neural Crest Cells as a Prerequisite for Head and Neck Development ( Figure 9-1 )
The formation of the head and neck strongly depends on the successful completion of neurulation (i.e., the formation of the central nervous system [CNS]). Early in week 4, parts of the most dorsal germ layer, the ectoderm, transform into the neural plate. The thickened epithelial cells, which form the neural plate, are located at and adjacent to the embryo’s craniocaudal axis. The neuronal plate invaginates along this axis, and the resulting neuronal folds separate from the ectoderm and fuse to form the neural tube, which in turn will develop into the CNS. The free edges of the remaining surface ectoderm fuse over the neural tube and thereby build a continuous layer, which later will differentiate into epidermis. In humans, the fusion of the neural folds starts at least at two discrete points along the craniocaudal axis and proceeds in cranial and caudal directions. The cranial portion of the neural tube, the future brain, closes completely on day 25. Failure of this fusion results in anencephaly and is incompatible with postnatal life. Anencephaly occurs with an incidence of approximately 1 in 4800 live births and is always accompanied by acrania, the partial or complete absence of the cranial vault ( Figure 9-2 ).
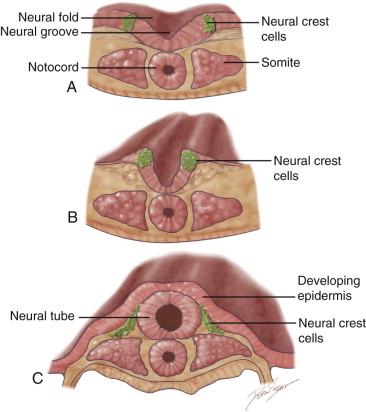
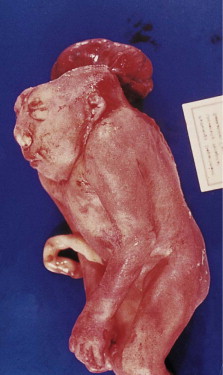
During the fusion of the neural folds into the neural tube, cells at the edge of the neural fold separate and form streaks of neural crest cells (NCCs) along the entire neural tube. NCCs undergo an epithelial-mesenchymal transition as they detach from the neural tube and migrate to various locations in the body. They form a vast array of structures, such as the ganglia of the autonomic nervous system, the enteric nervous system, and the adrenal medulla. In the head and neck region, NCCs give rise to much of the connective tissue in the craniofacial complex (skull, face, pharyngeal arches ). Therefore, neurocristopathies (i.e., the abnormal development of neural crest cells) often cause developmental defects that involve the craniofacial system (e.g., DiGeorge syndrome, Treacher Collins syndrome, and cleft formation).
Skull Development
Neurocranium and Viscerocranium ( Figure 9-3 )
Functionally, the cranium (skull) is composed of two parts: the neurocranium and the viscerocranium. The neurocranium surrounds and protects the brain and can be subdivided into the cranial base and the cranial vault. The viscerocranium comprises the facial skeleton and facilitates respiration and ingestion. The mesenchyme that forms the bones of the viscerocranium solely originates from NCCs, which migrate into the pharyngeal arches, whereas the material for bone formation in the neurocranium derives from both indigenous cephalic mesoderm and NCCs.
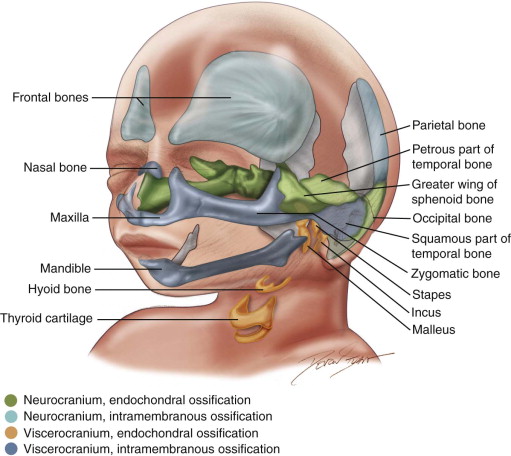
Intramembranous and Endochondral Ossification
In general, two forms of bone formation can be distinguished, both of which are found in skull development. In intramembranous ossification, mesenchymal tissue condenses and forms a highly vascularized membranous sheath. Osteoblasts differentiate from mesenchymal precursor cells and deposit osteoid (unmineralized bone matrix), which subsequently is calcified. In endochondral ossification, chondrocytes, which also differentiate from mesenchyme, initially form a cartilaginous model of the future bone. Starting at primary centers of ossification, osteoblasts then gradually replace the cartilage with bone tissue.
The bones that constitute the cranial base (i.e., the base of the occipital bone, the body of the sphenoid bone, petrous and mastoid parts of the temporal bone, and the ethmoid bone) are formed by endochondral ossification. This also holds true for several bones of the viscerocranium, such as the bones of the middle ear, the styloid process of the temporal bone, and the hyoid bone. The remaining bones of the viscerocranium—such as the mandible, the maxillary and zygomatic bones, and the squamous part of the temporal bone—are formed by intramembranous ossification. Similarly, the bones that shape the cranial vault (frontal and parietal bones, parts of the occipital bone) derive from intramembranous ossification.
The development of the temporomandibular joint starts at week 9 with the formation of the condylar process of the mandible, which is followed by the formation of the temporal portion of the joint (week 10). By week 14, the interarticular disc and the joint spaces have been formed.
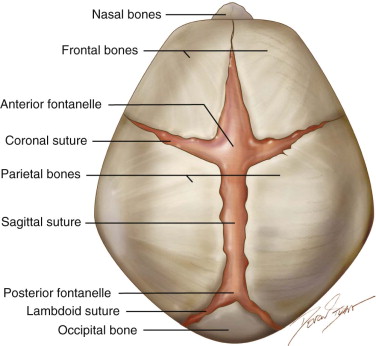
Sutures and Fontanelles ( Figure 9-4 )
The bones of the cranial vault are joined by syndesmoses. These sutures widen into larger fibrous areas, the fontanelles, whenever more than two bones meet. Sutures and fontanelles allow for molding of the head while passing through the birth canal. Moreover, the sutures are the sides of pre- and postnatal growth of the cranial bones, and whereas the fontanelles usually close within the first 2 years of prenatal life, most of the sutures only close completely later in adulthood. Coordinated growth of the central nervous system and its surrounding tissues (meninges, bones, and connective tissue) is essential for normal head development.
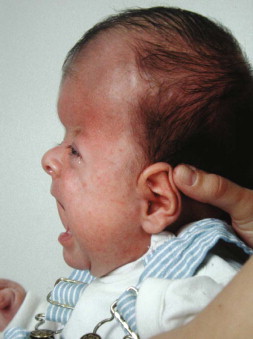
Craniosynostosis ( Figure 9-5 ), the premature fusion of one or more cranial sutures, results in restriction of the growing brain and craniofacial deformities due to compensatory growth in unaffected areas. The incidence for craniosynostosis is 1 in 2500 births and the defect can occur as an isolated event or as one component of various syndromes. Depending on which of the sutures fuse prematurely, the shape of the skull is altered. For example, scaphocephaly, a long and narrow skull, is the result of early fusion of the sagittal suture, whereas brachycephaly, a skull shortened in anteroposterior direction, is caused by premature fusion of both coronary sutures. Crouzon and Apert syndromes are two examples of syndromes associated with craniosynostosis of the coronary sutures. Mutations in a gene encoding for a fibroblast growth factor receptor cause both syndromes, which also feature deformation of other craniofacial structures, such as shallow orbits, hypertelorism, a hypoplastic midface region and maxilla, and mandibular prognathism.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


