Endosteal dental implants are highly successful, with numerous reports of success rates in the range of 89% to 99% for both individual and multiple implants. Attempts to improve these excellent results have been realized by altering the surfaces of titanium implants. Surface modifications have not only improved the long-term success of dental implants but have also allowed earlier loading with functional prostheses. These results have been achieved because the properties of biomaterial surfaces play a critical role in modulating the biologic response of the surrounding tissue. Bone morphogenetic proteins (BMPs) are a family of proteins that have been demonstrated experimentally and clinically to exert a positive effect on bone formation. Its commercial form, recombinant human BMP-2 (rhBMP-2), has undergone extensive scientific investigation and has received approval by the Food and Drug Administration (FDA) as a substitute for autogenous bone in maxillofacial and orthopedic applications. It has been demonstrated clinically to form bone in orthopedic sites (the spine and tibia) and the maxillofacial region, such as defects in the maxillary sinus floor and maxillary alveolar bone. Investigators have recently focused on using this bone induction factor in conjunction with dental and orthopedic implants as a means of accelerating bone healing around implants (osseointegration). A number of well-controlled animal studies have investigated the feasibility of using rhBMP-2 in conjunction with dental implants to accelerate the osseous response of the host and to improve healing in less than optimal sites such as type IV bone.
Surgical placement of dental implants initiates a sequence of cellular and molecular events that represents a combined response of wound healing and fracture repair. Proliferation plus differentiation of osteogenic cells (preosteoblasts) into osteoblasts follows activation of periosteal, perivascular, and endosteal lining cells under the influence of local and systemic growth factors. Osteoblastic differentiation initiates the production and mineralization of osteoid, followed by organization of the bone-implant interface. At times, deficiencies in the healing process may result in delayed healing and compromised implant integration. BMPs have undergone extensive study as factors capable of enhancing bone healing and as a substitute for autogenous bone grafts. BMP-2 has been shown to possess chemotactic properties, which results in the attraction of a variety of cellular elements to the site of implantation, and to be a mitogenic agent, whereby it increases the cellular population of important bone-forming cells. BMP-2 can also influence the differentiation and proliferation of mesenchymal progenitor cells into cells of a chondroblastic and osteoblastic lineage. The mode of action, bone-forming capacity, and safety of rhBMP-2 contained on an absorbable collagen sponge (ACS) have been extensively investigated and are well understood ( Box 24-1 ).
- •
Water soluble
- •
Diffuses away unless bound
- •
Degraded by local proteases
- •
Binds to collagen, hydrophilic surfaces
- •
Burst release may upregulate Noggins
- •
Recruitment of osteoclastic along with osteogenic cells
Scientific Investigations
In animal models, application of rhBMP-2/ACS resulted in induction of normal bone at the site of implantation. The induced bone undergoes remodeling, assumes the appropriate structure for the site with the defect, and displays anisotropic behavior in response to functional loads. Although earlier studies focused on the structure and biologic activity of this protein, over the past 14 years, additional investigations have used rhBMP-2 to accelerate and improve the quality and quantity of osseointegration. Since BMP is highly water-soluble, methods of delivering and retaining the protein have great physiologic significance. Essentially, rhBMP-2 can be combined with a material in one of two ways. Adsorption is a function of surface energy and is the process by which a protein is retained on the surface of a material by virtue of weak van der Waal forces or stronger covalent bonding. The adsorptive ability of a material is characterized by its wettability and can be improved by increasing the contact angle between the two materials. Incorporation is a different method of combining two substances. In this process, a protein is co-precipitated with another material (usually a ceramic) under physiologic conditions to preserve the bioactivity of the protein. Use of this technique to improve a material’s integration within biologic tissue is referred to as biomimetic technology. Both adsorption and incorporation can be used to transport and retain rhBMP-2 within the site of a defect. These processes can be used in conjunction with protein carriers or directly with a treated implant surface.
Carrier technology uses an inert material to transport and contain the protein within a defect site and thereby maintain sufficient physiologic concentrations of BMP for promotion of a clinically significant osteogenic response. Characteristics such as the rate of protein release and carrier degradation play important roles in material selection. Studies using different materials with different geometric properties and different methods of attaching the protein to the carrier have met with varying degrees of success. Several studies have investigated these different mechanisms of conveying rhBMP-2 to an implant site, and the results are described in the next section.
The Effect Of Carriers
In an effort to investigate the effect of BMP adsorbed onto a collagen sponge on implant osseointegration, Sykaras and colleagues created an edentulous region in the mandible of dogs and placed rhBMP-2/ACS into chambers of hollow basket, press-fit dental implants. One hundred four 3.5 × 8 mm implants were used, and the animals were divided into two groups to compare the response of bone formation at 2, 4, 8, and 12 weeks. In the treatment group, a concentration of 0.4 mg/mL of rhBMP-2 was placed into the apical hollow chamber of an implant just before insertion. The control group received only the implant without either a sponge or rhBMP-2 ( Fig. 24-1 ). The volume of rhBMP-2 delivered by each implant was 20 µg, and the concentration and dose were determined from previous studies on carrier release kinetics by the manufacturer. The collagen sponge retained approximately 95% of the rhBMP-2 during the initial impregnation and released the rhBMP-2 in two phases: an initial “burst” phase within hours of implantation and a second prolonged phase governed by the carrier (ACS) and its geometric characteristics.
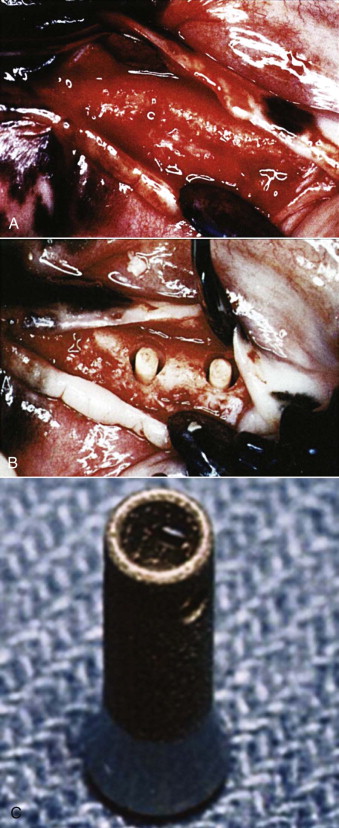
Uncomplicated healing followed the surgical procedures in all dogs. At the time of sacrifice, 23 implants (22%) were verified to be lost. There were no differences in failure rates between the rhBMP-2–treated implants and the controls (empty hollow baskets) ( P = .05). Histologic observations confirmed bone regeneration in both treated and control implants. Minimal bone formation was noted at 2 weeks in both groups, and the collagen sponge appeared to occupy most of the hollow chambers in the experimental group (rhBMP-2/ACS) without signs of degradation or infiltration by connective tissue. Branching organized trabeculae of woven bone were seen filling the defect in both groups. At 4 and 8 weeks, fibrous connective tissue was noted to engulf the collagen sponge, with fibroblasts infiltrating the sponge at the periphery. No evidence of local inflammation was found. Collagen sponge fragments were still evident at 12 weeks, thus suggesting slow degradation of the matrix and gradual replacement by bone. Along the side wall of the implant recipient site, increased amounts of host bone were observed in areas apposing the apical perforation (of the implant) in the rhBMP-2–treated sites.
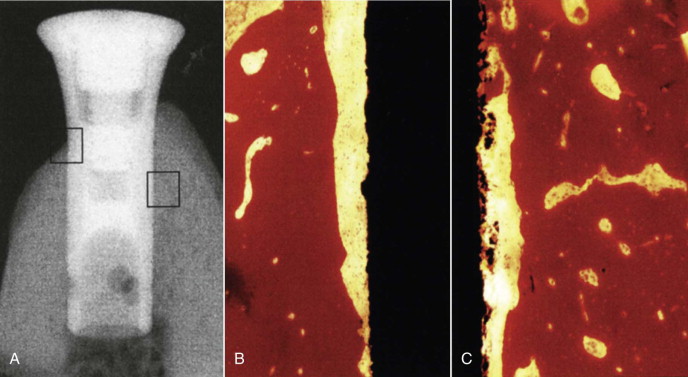
The histomorphometric results of assessment of bone-implant contact (BIC/%) of the hollow chamber surfaces in direct contact with bone are shown in Figure 24-2 . At 2 weeks, no statistical difference was found between the two groups. At 4 and 8 weeks, the experimental group showed significantly more bone apposition than did controls. The results of the BIC comparison between the two groups failed to show a statistical difference between the groups at 2 and 4 weeks, but BIC was higher at 8 and 12 weeks. Even though the treated implants exhibited increased bone fill around the hollow cylinders, the area of regenerated bone was statistically lower than the area in the initial host site. This incongruous finding may be due to the walls of the titanium implant preventing the osteoblast progenitor cells from penetrating into the hollow portion of the implant. Another study used histomorphometric and radiographic imaging to evaluate the effect of rhBMP-2 on osseointegration. It was determined that rhBMP-2/ACS confined to the hollow implant chamber does not improve osseointegration along the implant’s outer surface, probably because the chamber walls limit diffusion of the signaling protein. Radiographic evaluation resulted in an overestimation of BIC and poor correlation with histomorphometric data.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


