Introduction
The aim of this experimental study was to evaluate the effects of systemically applied zoledronic acid on bone regeneration in response to expansion of the sagittal suture and relapse in rats.
Methods
Thirty-six male Wistar rats were divided into 3 groups. In the first and second groups, saline solution was given subcutaneously after expansion, and the retention periods lasted 14 and 7 days, respectively. In the third group, 0.1 mg of zoledronic acid was diluted with saline solution and given subcutaneously after expansion; the retention period lasted for 7 days. Expansion and relapse amounts were measured by using computed tomography. After the retention period, 6 rats from each group were killed for histologic and immunohistochemical assessments. The other 6 rats from each group were used for observation of the relapse.
Results
The histologic evaluation showed that, in groups 1 and 2, the numbers of osteoblasts were less than observed in group 3. When scores of staining intensity were compared, immunoreactivities were statistically significantly increased in group 3 compared with groups 2 and 1. Statistically significant differences were found when the relapse percentages were compared between the groups ( P <0.05). The smallest relapse occurred in group 3.
Conclusions
Zoledronic acid has positive effects on bone formation in the sagittal suture in response to expansion and decreases the relapse ratio after expansion in rats.
The malocclusions caused by a maxillary transverse deficiency, such as Class I with crowding, Class II with a V-shaped arch, and Class III with a small maxilla in growing patients, are often treated with rapid palatal expansion. After the desired expansion, the suture undergoes remodeling with bone formation, resorption, and fiber rearrangement. This continues until the architectural environment achieves equilibrium. It is well documented that, even after a retention period, the expanded maxillary dental arch has a strong tendency to rebound to its previous form. The literature reported the percentages of relapse after retention from 0% to 45%. Although a 6-month retention period was applied conventionally, Da Silva Filho et al showed that the midpalatal suture was completely ossified from the anterior nasal spine area to the posterior nasal spine area after the retention phase, which was 8 to 9 months postexpansion. It would be potentially beneficial, therefore, to accelerate bone formation in the midpalatal suture after expansion to prevent relapse of the arch width and to shorten the retention period. To maintain the maxillary expansion, stimulation of bone formation in the expanding suture with low-power laser irradiation and transforming growth factor-β1 or vitamin D analog injection has been previously reported.
Bisphosphonates are now the most widely used drugs for the treatment of some metabolic bone diseases, such as Paget’s disease, osteogenesis imperfecta, fibrous dysplasia, Gaucher’s disease, malignant hypercalcemia, and osteoporosis. Bisphosphonates can bind to hydroxyapatite crystals in a mineralized bone matrix and make the bone more resistant to osteoclasts, inhibit differentiation of bone marrow precursors into osteoclasts, inhibit osteoclast function by interfering with the mevalonate pathway of cholesterol biosynthesis, and induce apoptosis of osteclasts. In recent years, it has became known that bisphosphonates not only restrict osteoclastic activity, but also show osteo-conductive and osteo-inductive effects by increasing osteoblastic activity.
The developments that concern bisphosphonates have received interest in the dentistry field, and bisphosphonates have been used in some dental studies, such as those concerning implant surgery, periodontitis, and alveolar defects. Clinical and radiologic measurements showed that bisphosphonates increase early bone formation rates around dental implants and noticeably increase bone mineral density in the treatment of periodontal defects. Studies have shown that bisphosphonates can reduce bone loss in animal models of experimentally induced and naturally developing periodontitis. Also, the effect of bisphosphonate therapy on periodontitis was assessed in human studies. Six months of treatment with bisphosphonates produced improvement in alveolar bone crest height in patients with type 2 diabetes and established periodontitis. Although bisphosphonate therapy improves the outcome of conventional periodontal treatment, as the duration of bisphosphonate use increases, its protective effect appears to decrease.
Zoledronic acid (ZA), a third-generation, nitrogen-containing heterocyclic imidazole bisphosphonate, has been found to be a more potent inhibitor of bone resorption than other bisphosphonates that are currently available.
The aim of this study was to evaluate the effects of systemically administered ZA on osteoblastic activity and relapse in the sagittal sutures of rats after expansion. To eliminate the effects of occlusal forces and mastication, this study was designed at the rats’ sagittal sutures.
Material and methods
Ethical approval (B.30.2.Cum.0.01.00.00-50/146) was obtained for this study from the Animal Research Ethics Committee at the Comhuriyet University School of Medicine in Sivas, Turkey.
A total of 36 male Wistar rats with a mean weight of 200 ± 10 g were divided into 3 groups of 12 animals each. All animals were kept in separate cages in a 12-hour light and dark environment at a constant temperature of 23°C and fed an ordinary, solid diet and water ad libitum. Body weight was measured every day during the entire experimental period.
Suture expansion was carried out for 7 days on all animals by using an expansion spring made of 0.5-mm diameter stainless steel wire (Dentaurum, Pforzheim, Germany) with 2 helices ( Fig 1 , A ).
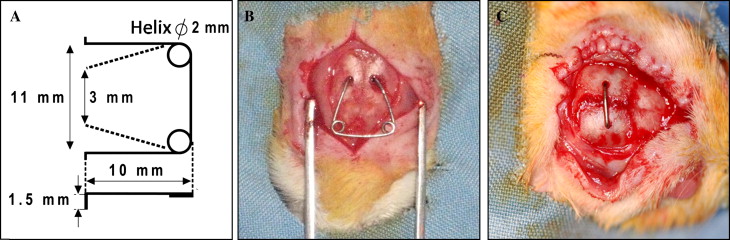
The rats were anesthetized with intramuscular injections of a combination of 90 mg per kilogram of ketamine hydrochloride (Ketalar-Eczacbaş, Istanbul, Turkey) and 3 mg per kilogram of xylazine (Rompun-Bayer, Leverkusen, Germany). The hair was shaved, the skin was cleaned, and the area to be operated on was disinfected with povidone-iodine before the surgical procedure (Batticon-Adeka, Istanbul, Turkey). A 1.5 to 2 cm midsagittal incision was made anteroposteriorly through the scalp to expose the sagittal suture. Subsequently, 2 holes were opened symmetrically in the parietal bones with a physiodispenser under saline-solution irrigation. The distance between the 2 holes on opposite sides of the suture was 3 mm. The expansion spring was calibrated in advance to exert an initial expansion force of 120 g. Finally, the expansion spring was placed into the holes, and the scalp was sutured over the spring ( Fig 1 , B ).
After the sutural expansion period, the expansion springs were removed from all rats, and retention appliances were placed into the holes under general anesthesia with the same surgical procedure. Groups 1, 2, and 3 underwent 14, 7, and 7 days of mechanical retention, respectively, with a retention appliance ( Fig 1 , C ). A physiologic saline solution (5 mg/kg, 0.9% sodium chloride) was injected subcutaneously into the animals in groups 1 and 2, which were the control groups. A single dose of 0.1 mg per kilogram of ZA (Zometa, Novartis, East Hanover, NJ) dissolved in 5 mg per kilogram of physiologic saline solution was injected subcutaneously into group 3, which was the study group. To compare the ZA-injection rats with those that underwent identical and longer retention periods, 2 control groups were included. Groups, interventions, and retention periods are shown in Table I .
| Group | Intervention | Retention period (d) |
|---|---|---|
| 1 | Expansion + NaCl | 14 |
| 2 | Expansion + NaCl | 7 |
| 3 | Expansion + ZA | 7 |
At the conclusion of the retention period, 6 rats from each group were killed with 200 mg per kilogram of sodium pentobarbital (Pentothal, Abbot, North Chicago, Ill) for histologic and immunohistochemical assessment. The other 6 rats from each group were used for observation of relapse. The retention appliances were removed under general anesthesia, and the rats underwent a 7-day relapse period. At the end of the experimental period, the animals were killed under general anesthesia with 200 mg per kilogram of sodium pentobarbital (Pentothal, Abbot).
The distance between the holes was measured by using computed tomography (CT). The axial CT images of the rats were taken with the Brilliance CT System (Philips Medical Systems, Eindhoven, The Netherlands) in a standard position at Cumhuriyet University, Faculty of Medicine, Department of Radiology, with a tilt of 0°, thickness and table feed of 0.8 mm, and original screen resolution of 512 × 512 matrix with 16 bits. The CT data were transferred directly from the CT scanner to a personal computer as raw data sets without the loss of signals. After the transfer of the CT data to the personal computer, the distance between the holes was measured with the MX View Workstation program (Philips Medical Systems, Cleveland, Ohio) ( Fig 2 ). CT measurements were taken at the beginning (T1) and the end of the expansion period (T2), after the retention period (T3), and at the end of the follow-up period (T4).
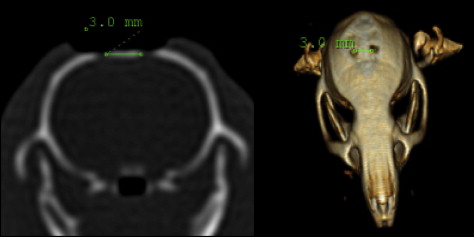
The relapse ratio or the rate of decrease in the distance was calculated according to the following equation: (T4 – T3) / (T3 – T1) × 100.
Cranium samples were dissected and fixed in 10% formalin solution for 24 hours. After fixation, the craniums were decalcified by using ethylenediaminetetraacetic acid (0.1 mol/L,pH = 7.1) solution. During decalcification, the solution was changed every second day at +4°C for 3 weeks. After decalcification, the operation areas were removed from the cranium with a scalpel ( Fig 3 ). Routine paraffin embedding procedures were used. In brief, after decalcification, tissue samples were dehydrated in a graded ethanol series, cleared in xylene, and embedded in paraffin wax; then 5-μm thick sections were cut. All formalin-fixed, paraffin-embedded tissue blocks were evaluated at the Research Laboratory of Histology and Embryology, Celal Bayar University, in Manisa, Turkey. The tissue blocks were chosen carefully after histologic assessment of sections stained with hematoxylin and eosin (Surgipath, 01562E, 01602E, Peterborough, United Kingdom).
For immunohistochemical staining, sections were incubated at 60°C overnight and then held in xylene and rehydrated through a series of ethanol solutions. Sections were washed with distilled water and phosphate-buffered saline solution (PBS; P4417, Sigma-Aldrich, St Louis, Mo) for 10 minutes and then treated with 0.1% trypsin (Zymed, South San Francisco, Calif) at 37°C for 10 minutes and washed with PBS. Sections were delineated with a pen (S2002, Dako, Glostrup, Denmark) and incubated in a solution of 3% hydrogen peroxide (TA-015-HP, Dako) for 5 minutes to inhibit endogenous peroxidase activity. After washing in PBS, the sections were incubated with a nonimmune serum (Ultra V block, cat. No: TA-125-UD, Lab Vision, Fremont, Calif) for 1 hour, and then sections were incubated with primary antibodies: monoclonal mouse anti-osteonectin (33-5500, Zymed), monoclonal mouse anti-osteocalcin (33-5400, Zymed), monoclonal mouse anti-VEGF (SC-7269, Santa Cruz Biotechnology, Inc, Santa Cruz, Calif), polyclonal rabbit anti-TGF-β (SC-146, Santa Cruz Biotechnology, Inc), 1:100 dilution, 1 hour at 4°C in a humidity chamber. The sections were washed 3 times for 5 minutes each with PBS, followed by incubation with biotinylated secondary antibody and then with streptavidin conjugated to horseradish peroxidase in PBS for 30 minutes each (Histostain-plus-Peroxidase kit, 85-9043, Zymed). After washing 3 times with PBS, the sections were incubated with di amino benzidine (Dako) for 5 minutes for immunostaining.
After washing with distilled water, the sections were counterstained with Mayer’s hematoxylin (Richard-Allan Scientific, Kalamazoo, Mich) and washed with distilled water. The sections were mounted with a mounting medium and were observed with a BX 40 bright-field microscope (Olympus, Tokyo, Japan). The red-brown precipitate indicated positive findings for the primary antibodies. The negative control samples were processed identically; instead of primary antibodies, the same types of immunoglobulin G were used. Two observers (S.I.), blinded to the clinical information, evaluated the staining scores independently, and no statistical interobserver difference was found. The mean values of the immunohistochemical staining intensities were graded semiquantitatively as mild (+), moderate (++), or strong (+++).
In 40-times magnification, the numbers of active osteoblasts were scored as + (1-10), ++ (11-20), or +++ (>20). The widths of the vessels were scored as + (narrow), ++ (moderate), or +++ (wide).
Statistical analysis
The data were analyzed by using SPSS for Windows (version 13.0, SPSS, Chicago, Ill). The level of significance was set at P <0.05. To evaluate the distance measurements in the T1, T2, and T3 periods and compare them between groups, the Kruskal-Wallis test was used. To assess the differences in each group over the various periods, the Friedman and Wilcoxon tests were used.
To evaluate relapse after the retention period (T3-T4), the Kruskal-Wallis test and the Mann-Whitney U test were used.
Histologic and immunohistochemical measurements (T3) were evaluated with the Kruskal-Wallis and Mann-Whitney U tests.
Results
Placement of the expansion springs and retention appliances caused temporary reductions in body weight (–5%). Suture separation was successfully achieved with the expansion spring.
There were significant differences between the different time points (T1, T2, T3) on the CT measurements in all groups ( P <0.05) for the expansion amounts in the groups. In addition, there were significant differences in the distances between the T1-T2 and T1-T3 periods ( P <0.05). However, there was no significant change between T2 and T3 ( P >0.05). Table II shows the average measurement and standard deviations at T1, T2, and T3.
There were no significant differences between the groups in the CT measurements at T1. There were no significant differences in the amount of expansion (T1-T2) between the groups. Furthermore, the amount of expansion was maintained after the retention period in all groups (T2-T3) ( Table III ).
There were significant differences when the relapse percentages between the groups ( P <0.05) were compared. The differences in relapse amounts were significant between groups 1 and 2, groups 1 and 3, and groups 2 and 3 ( Table III ). The smallest relapse percentage was observed in group 3.
The histologic and immunohistochemical appearances of all groups are shown in Figures 4 through 8 .
In the examination of the sagittal suture samples stained with hematoxylin and eosin from group 1 (7-day expansion and 14-day retention) under the light microscope, it was observed that (++/+++) active osteoblasts were localized along the suture and the width of the blood vessels ( Fig 4 ). In this group, moderate (++) osteocalcin and osteonectin immunoreactivities were observed in osteoblasts, and moderate/strong (++/+++) immunoreactivities were observed in the suture connective tissues ( Figs 5 and 6 ). In this group, moderate (++) to moderate/strong (++/+++) VEGF and TGF-β immunoreactivities were observed in active osteoblasts and suture connectives tissue by using an indirect immunohistochemistry method ( Figs 7 and 8 ).
In the examination of the sagittal suture samples stained with hematoxylin and eosin from group 2 (7-day expansion and 7-day retention) under the light microscope, it was observed that (++) active osteoblasts were localized along the suture and the moderate-width blood vessels ( Fig 4 ). In this group, minimal/moderate (+/++) osteocalcin and osteonectin immunoreactivities were observed in osteoblasts, and moderate (++) immunoreactivities were observed in the suture connective tissues by using an indirect immunohistochemistry method ( Figs 5 and 6 ). In this group, moderate (++) VEGF and TGF-β immunoreactivities were observed in active osteoblasts and suture connective tissues ( Figs 7 and 8 ).
In the examination of the sagittal suture samples stained with hematoxylin and eosin from group 3 (7-day expansion, ZA, and 7-day retention) under the light microscope, it was observed that (+++) active osteoblasts were localized along the suture and the width of the blood vessels ( Fig 4 ). In this group, strong (+++) osteocalcin and osteonectin immunoreactivities were observed in osteoblasts, and minimal/moderate (+/++) immunoreactivities were observed in suture connective tissues by using an indirect immunohistochemistry method ( Figs 5 and 6 ). In this group, strong (+++) VEGF and TGF-β immunoreactivities were observed in active osteblasts and suture connective tissues ( Figs 7 and 8 ).
When scores of staining intensity were compared, a statistically significant increase was found in group 3 compared with groups 2 and 1 ( Table IV ).



