Introduction
The objective of this 3-arm parallel randomized clinical trial was to compare the effectiveness of temporary anchorage devices (TADs), Nance button palatal arches, and headgear for anchorage supplementation in the treatment of patients with malocclusions that required maximum anchorage. This trial was conducted between August 2008 and February 2013 in 2 orthodontic departments in the United Kingdom.
Methods
The study included 78 patients (ages, 12-18 years; mean age, 14.2 years) who needed maximum anchorage. Eligibility criteria included no active caries, exemplary oral hygiene, and maximum anchorage required.
Outcome
The primary outcome was mesial molar movement during the period in which anchorage supplementation was required. The secondary outcomes were duration of anchorage reinforcement, number of treatment visits, number of casual and failed appointments, total treatment time, dento-occlusal change, and patients’ perceptions of the method of anchorage supplementation.
Randomization
Treatment allocation was implemented by contacting via the Internet the randomization center at the University of Nottingham, Clinical Trials Unit. The randomization was based on a computer-generated pseudo-random code with random permuted blocks of randomly varying size.
Blinding
A research assistant who was blinded to the group allocation recorded all data.
Intervention
The patients were randomly allocated to receive anchorage supplementation with TADs, a Nance button on a palatal arch, or headgear. They were all treated with maxillary and mandibular preadjusted edgewise fixed appliances with 0.022-in slot prescription brackets. They were followed until orthodontic treatment was complete.
Results
Seventy-eight patients were randomized in a 1:1:1 ratio among the 3 groups. The baseline characteristics were similar in the groups, and they were treated for an average of 27.4 months (SD, 7.1 months); 71 completed orthodontic treatment. The data were analyzed on a per-protocol basis and showed no differences in the effectiveness of anchorage supplementation between TADs, Nance button palatal arches, and headgear. Compared with headgear, the average mesial movements of the maxillary right molar were 0.62 mm (−0.32 to 1.55 mm) with the Nance and −0.58 mm (−1.53 to 0.36 mm) with TADs; the maxillary left molar was moved −0.09 mm (−1.00 to 0.83 mm) with the Nance and −0.96 mm (−1.89 to −0.04 mm) with the TADs. Peer assessment rating scores were significantly better with the TADs than in the headgear and Nance groups. The patient questionnaires showed that comfort levels on placement of the TADs and the Nance were similar. Headgear was more troublesome and less popular with the patients.
Conclusions
There was no difference in the effectiveness between the 3 groups in terms of anchorage support. There were more problems with the headgear and Nance buttons than with the TADs. The quality of treatment was better with TADs. As a result, TADS might be the preferred method for reinforcing orthodontic anchorage in patients who need maximum anchorage.
Trial registration
ClinicalTrials.gov Identifier: NCT00995436.
Protocol
The protocol was published on the above site before the trial commencement.
Funding
The British Orthodontic Society Foundation funded the study and American Orthodontics provided all the TADs and associated equipment.
In this article, we present the results of a randomized controlled trial investigating the effectiveness of methods of anchorage reinforcement for orthodontic treatments requiring maximum anchorage. When the evidence base underpinning this type of treatment is critically examined, the level of evidence is not high. For example, when we reviewed recently published trials as part of a Cochrane Review we found 7 publications. Of these, 1 study suggested that headgear and midpalatal implants were equally effective in providing anchorage, whereas another large study found in favor of surgically assisted anchorage. Interestingly, both studies used palatally placed osseointegrated surgical anchorage devices. Two further studies evaluated temporary anchorage devices (TADs), comparing them with conventional anchorage, such as headgear, palatal arches, and banding of second molars. These studies concluded that TADs were more effective than other methods of anchorage supplementation.
When we consider any form of orthodontic treatment, it is essential to study the patients’ perceptions, since their values can differ between treatment methods. Unfortunately, this has only been considered in a few studies evaluating anchorage supplementation. This information has been confined to the patients’ perception of pain or discomfort associated with implant placement or removal. They reported that the placing and removal of midpalatal implants and onplants are uncomfortable, requiring extensive local anesthesia and often postsurgery analgesia, compared with the relatively simple procedures of placement and removal of TADs.
We therefore decided to investigate the effectiveness of 3 methods of anchorage supplementation, with a group of patients defined as needing maximum anchorage, and report on both orthodontists’ and patients’ values.
We tested the hypothesis that there is no difference in the effects of TADs, headgear, and Nance button palatal arches when used to reinforce orthodontic anchorage with respect to (1) the amount of molar tooth movement, (2) the duration of treatment, (3) the number of treatment visits, (4) the total treatment time, (5) dento-occlusal changes (peer assessment rating [PAR] index), and (6) the patients’ perceptions of the treatment.
Material and methods
Trial design
This 3-arm parallel group randomized clinical trial had a 1:1:1 allocation ratio.
Participants, eligibility criteria, and settings
Participants were recruited at 2 hospital orthodontic departments in the United Kingdom, Chesterfield Royal Hospital and Royal Derby Hospital, and treated by 2 clinicians (J.S. and A.M.), both of whom have wide experience with the treatment methods. The clinicians were salaried hospital employees, and all treatments were provided within the United Kingdom’s National Health Service at no direct cost to the patient or family. The study was approved by the Central Research Ethics Committee and the research and development departments at Chesterfield Royal Hospital and Royal Derby Hospital National Health Service trusts. A data-monitoring committee was established, and annual reports were submitted to this committee throughout the study to reassure them that progress was being made and that any untoward effects were reported. The trial was registered at ClinicalTrials.gov Identifier: NCT00995436, and the protocol was published on that site before the trial. We followed the guidelines in the declaration of Helsinki.
The study was carried out with 78 patients. To be included, patients had to be between 12 and 18 years old. The operators had assessed them as needing maximum anchorage. This was defined as “no mesial movement of the molars during the period of anchorage supplementation.” No attempt was made to achieve distal molar movement because clinically this was not required.
The exclusion criteria for the study were patients who (1) required functional appliance therapy or orthognathic surgery, (2) had previous orthodontic treatment or extractions, (3) had hypodontia of more than 1 tooth per quadrant, (4) had craniofacial syndromes or clefts, and (5) had poor dental health precluding orthodontic treatment.
Interventions
All patients were fitted with McLaughlin, Bennett, Trevisi prescription (American Orthodontics, Sheboygan, Wis) maxillary and mandibular preadjusted edgewise fixed appliances. During the initial phase of treatment, we derotated the molars in all 3 treatment groups. This involved bracket placement on all premolars and molars in the maxillary buccal segments and working through 3 archwires (0.016-in Sentalloy [Dentsply, GAC, Bohemia, NY], 0.018 × 0.025-in Neo-Sentalloy, and 0.019 × 0.025-in stainless steel [American Orthodontics]) until the molars were aligned ( Fig 1 ). At this point, the first data collection (DC1) records were taken.
We observed the following treatment protocols.
For the headgear patients, this was a pragmatic trial, and all operators determined the design of the headgear according to their current treatment protocols. They placed 250 g of force per side on the headgear bow and requested at least 100 hours of headgear wear per week from the patients. We asked them to fill in a diary of headgear wear throughout treatment. The clinician checked both the headgear force and the compliance with headgear charts at each visit. Extractions were performed once the headgear was fitted.
For the Nance button patients, molar bands were fitted, and an alginate impression was taken over the bands; a Nance button on a 1.0-mm stainless steel palatal arch was made by an orthodontic technician. A large Nance button was used to cover the entire vertical part of the hard palate. This was fitted 1 week later, and any required extractions were arranged.
For the TADs patients, the maxillary and mandibular fixed appliances were placed, and arrangements were made for the necessary extractions before the TADs were placed. On the sides requiring anchorage supplementation, 8 × 1.6-mm TADs (American Orthodontics) were placed under local anesthesia, usually at the junction of the attached gingivae with the reflected mucosa and mesially to the maxillary molars. The TADs were placed before any retraction force was placed on the canines. Retraction was carried out with nickel-titanium springs ligated directly from the canine to the TAD to produce 90 to 100 g of force.
All patients were treated similarly, with a standard archwire sequence (0.016-in Sentalloy, 0.018 × 0.025-in Neo-Sentalloy, and 019 × 0.025-in stainless steel), and the canines were retracted to achieve a Class I canine relationship before complete overjet reduction and space closure. Anchorage supplementation was discontinued once the canines were Class I and there was sufficient space in the maxillary arch to complete the correction of the malocclusion. At this point, the operator judged that no further anchorage supplementation was needed; therefore, the headgear was stopped, and the TADs or the acrylic Nance button was removed.
Outcomes, primary and secondary
The primary outcome measure in this study was movement of the molars. The following secondary outcome measures were collected from the patients’ treatment records.
- 1.
The process of treatment (number of attendances, duration of treatment, number of missed and canceled appointments, and any emergency appointments).
- 2.
The dento-occlusal outcome of treatment using the PAR index with the United Kingdom weightings. Calibrated dental technicians, blinded to treatment allocation, performed this.
In addition, the patients were given questionnaires about the comfort and discomfort levels of placement and removal of both TADs and Nance palatal arches during the week after the procedure. The headgear patients only completed a questionnaire about their clinical experiences.
There were no outcome changes after commencement of the trial.
The data were collected at the following time points: at the start of full arch treatment when anchorage supplementation was provided (DC1), when anchorage supplementation was no longer required (DC2), and when active orthodontic treatment was complete (DC3). The following were collected: (1) maxillary and mandibular silicone impressions (DC1, DC2, DC3); (2) photographs, 4 extraoral and 5 intraoral (DC1, DC3), and intraoral (DC2); (3) orthopantomogram images (DC1, DC3); (4) TADs questionnaire (2 weeks after placement and 2 weeks after removal); (5) Nance questionnaire (2 weeks after placement and 2 weeks after removal); (6) headgear questionnaire (2 weeks after headgear was stopped); and (7) PAR scores (DC1, DC3).
Data collected from patient notes included (1) number of attendances; (2) number of visits from DC1 to DC2 and from DC2 to DC3; (3) duration of overall treatment; (4) number of missed or canceled appointments; and (5) frequency and reason for additional appointments for appliance breakage.
To make sure that the study models taken at DC2 did not provide information on the treatment allocation, we did the following. In the Nance group, the palatal arch was cut away from the bands, and the acrylic Nance button removed 2 weeks before the DC2 records were taken. This allowed any inflammation of the palatal tissues to subside and normal anatomy to reestablish. In the TADs group, the TADs were also removed before the impressions were taken.
To allow 3-dimensional (3D) digital scans of the models to be produced, the study models were sent to Bioprecision Diagnostics (Yeovil, Somerset, United Kingdom), where they were scanned by a 3Shape scanner ( www.3shape.com ) using 3Shape Scanserver and 3Shape ScanItOrthodontics software packages (3Shape, Copenhagen, Denmark). Surface shape measurements of the models were recorded through triangulation, and the computer then converted this information into a 3D polygon mesh. The detailed scans were trimmed using another software (Rhinoceros CAD; McNeel Europe, Barcelona, Spain; www.rhino3d.com ).
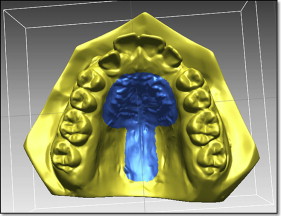
We measured tooth movement using Rapidform 2006 (Geomagic, Rock Hill, SC) software that allowed superimposition of the DC1 and DC2 maxillary 3D digital models, using the iterative closest point algorithm. This is a minimization routine, whereby many iterations of the superimposition process are performed within 6 degrees of freedom. On the pitch, yaw, and roll axes, the models can either translate or rotate, until the computer successfully minimizes the sum of the squares of the Euclidean distances between corresponding points on the 3D digital models at DC1 and DC2. The computer is directed to base the superimposition on an area of known stability common to both the DC1 and DC2 models; we selected this as the blue mushroom-shaped area based on the palatal rugae and a stable area of the hard palate shown in Figure 2 . The precision of the superimposition was assessed by the color of the models; the blue part of the spectrum indicates almost perfect superimposition of the 2 areas that are common to both digital models ( Fig 3 ).
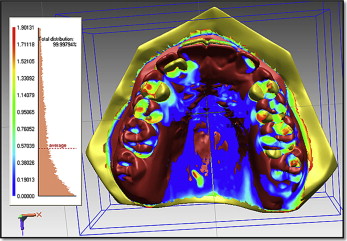
The DC1 maxillary molar outlines were then selected ( Fig 4 ). The software constructed a DC1 molar shell, and this molar shell was superimposed on the DC2 molar occlusal surface, using best-fit algorithms. Tooth movement was calculated by measuring the difference in position of the DC1 and DC2 centers of mass ( Fig 5 ).
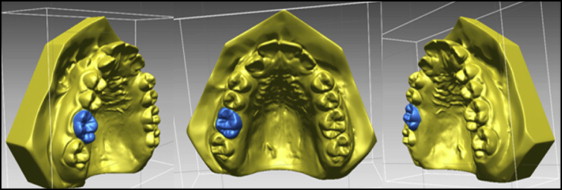
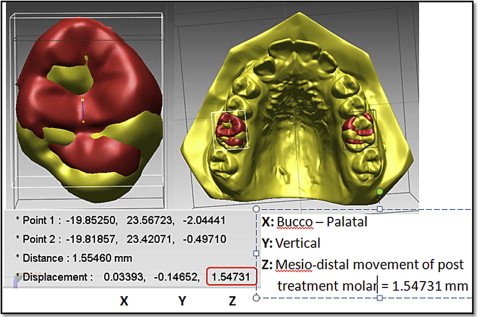
A research assistant (R.G.) who was blinded to treatment allocation made the superimpositions and measurements of tooth movement.
Sample size calculation
Calculation of the sample size was based on the ability to detect a clinically relevant difference in anchorage loss of 1.5 mm between 2 of the treatment groups. The expected standard deviation of mesial molar movement was taken from the study of Luecke and Johnstone, who investigated molar movement in premolar extraction patients when anchorage was supplemented with headgear. The calculation indicated that for a study with a power of 80% and an alpha of 0.05, we required 21 participants per group. We assumed a dropout rate of 20%, based on a previous study suggesting that a minimum of 75 patients was required. nQuery Advisor statistical software (Statistical Solutions, Boston, Mass) was used for the actual calculation.
Interim analyses and stopping guidelines
No interim analyses were planned, and the data-monitoring committee was happy with our progress throughout the study.
Randomization (random number generation, allocation concealment, implementation)
Randomization (random number generation, allocation concealment, implementation) was done as follows. When we identified patients who satisfied the inclusion criteria, we asked them to take part in the trial. Once informed consent was obtained from either competent patients or their parents, the orthodontist accessed the University of Nottingham Clinical Trials Unit randomization service ( http://www.ctsu.nottingham.ac.uk/0822/login.asp ) to obtain a treatment allocation. This ensured separation of the recruitment and randomization processes.
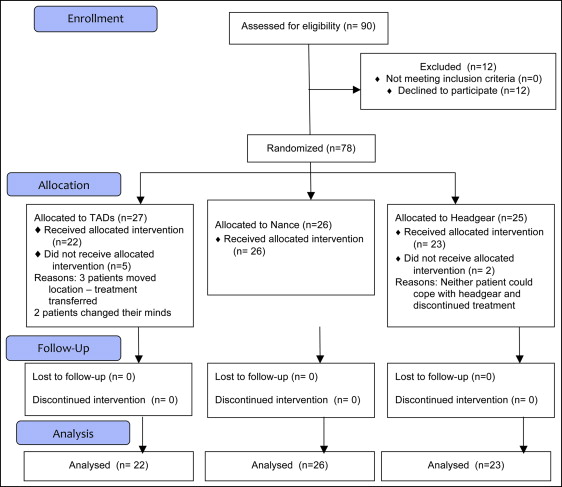
The randomization was based on a computer-generated pseudorandom code with random permuted blocks of randomly varying sizes. The sequence was held on a secure server according to standard operating procedures. When the patient and the parent had consented to be in the study, the randomization center at the University of Nottingham Clinical Trials Unit was contacted. Demographic data were entered; after confirmation of the veracity of the data, the group allocation was indicated. Twenty-seven patients were randomized to the TADs group, 26 to the Nance group, and 25 to the headgear group. At DC2, we analyzed 22 TADs patients, 26 Nance patients, and 23 headgear patients ( Fig 6 ).
Blinding
The clinicians and the patients were blinded to the allocation sequence; however, it was impossible to blind them to the treatment method. Assessment was blind because it was impossible to distinguish between the groups, since the Nance and the TADs had been removed before the DC2 records were taken.
Statistical analysis
A double determination was performed on 20 pairs of superimposed digital models. The study models were randomly picked and analyzed at 2 time points 4 weeks apart.
Bland-Altman plots, intraclass correlation coefficients, and paired t tests were used to assess reliability.
Summary statistics were calculated for the data at the start and finish points of the study. We checked the variances of the molar movements for normality; when they were found to be normally distributed, parametric tests were appropriate.
A per-protocol analysis was performed. All patients in the study either were included in the data analysis or, if they had dropped out, were reported on individually.
The data were analyzed with analysis of covariance (ANCOVA), with headgear as the reference group and molar movement at DC2 as the dependent variable. The independent variables were the treatment groups (Nance, headgear, and TADs). Because the groups were unbalanced in their sex distributions after randomization, we also fitted sex as a covariate to adjust for the baseline scores.
A similar analysis was carried out for the secondary outcome measures; the dependent variables were total treatment time, total number of visits, number of missed and canceled appointments, and dento-occlusal changes as measured by PAR scores. When it was relevant, the pretreatment PAR score was fitted as a covariate.
The SPSS software package was used (version 21; SPSS, Chicago, Ill), and statistical significance was set at the 5% level.
Results
Participant flow
Ninety patients were initially informed about the study, and 12 declined to enter. Three did not want to wear headgear, 3 did not want the Nance button, but only 1 patient did not want to take part because he or she was unhappy at “the thought of TADs.” The other 5 patients had personal reasons for not wanting to take part in a research study. All patients who declined were offered alternative treatments.
Of the 78 patients enrolled, 27 were allocated to TADs (16 girls, 11 boys; average age, 14.15 years; SD, 1.25 years), 26 to Nance (7 girls, 19 boys; average age, 14.14 years; SD, 1.48 years), and 25 to headgear (14 girls, 11 boys; average age, 14.38 years; SD, 1.67 years). The first patient was enrolled on August 5, 2008, and the final patient was enrolled 28 months later on December 22, 2010. All treatments were completed by February 2013. Two headgear patients and 5 TADs patients dropped out during the treatment period. The headgear patients were unable to cooperate with their treatment. The patients allocated to the TADs group stopped treatment for various social and domestic reasons. No patient who dropped out had reached the stage of having the TADs placed.
The flow of patients through the study is shown in Figure 6 . No dropout patient reached a stage where the DC2 records could be taken.
Baseline data
At baseline, information regarding age, sex, starting PAR score, and maxillary prominence was collected. Summary statistics for the patients are included in Table I . The baseline characteristics were similar in the 3 groups at the start of treatment. There was a lower proportion of girls in the Nance group compared with the other 2 groups.



