In this article, we summarize the most clinically relevant findings of our recently updated Cochrane systematic review into the treatment of Class II Division 1 malocclusion.
Methods
A systematic review of the databases was performed to identify all randomized controlled trials evaluating early treatment with functional appliances to correct Class II Division 1 malocclusion.
Results
Three early treatment studies with data from 353 participants were included in this review. The results showed no significant difference for any outcomes, except new incidence of incisor trauma, which was significantly less for the early treatment group. The risk ratio analysis for new incisor trauma showed that providing early treatment reduced the risk of trauma by 33% and 41% in the functional and headgear groups, respectively. However, when the numbers needed to treat were calculated, early treatment with functional appliances prevents 1 incidence of incisal trauma for every 10 patients (95% CI, 5-174), and headgear treatment prevents 1 incidence of incisal trauma for every 6 patients (95% CI, 3-23).
Conclusions
Orthodontic treatment for young children, followed by a later phase of treatment when the child is in early adolescence, appears to reduce the incidence of new incisal trauma significantly compared with treatment that is provided in 1 phase when the child is in early adolescence. However, these data should be interpreted with caution because of the high degree of uncertainty. There are no other advantages in providing 2-phase treatment compared with 1 phase in early adolescence.
In this article, we outline and discuss the most clinically relevant findings of our recently updated Cochrane systematic review into the treatment of Class II malocclusion. This form of malocclusion affects nearly a quarter of 12-year-olds in the United Kingdom and 15% of 12- to 15-year-olds in the United States. As a result, correcting Class II malocclusion is a common treatment performed by orthodontists.
There has been extensive research into Class II treatment, and this was summarized in the previous version of our Cochrane review, when we concluded that “early treatment of Class II malocclusion resulted in limited advantage when compared to providing treatment in one stage during adolescence.” Despite this high level of evidence, these conclusions are still thought to be controversial because the results of nonrandomized investigations do not always agree with the results of the trials.
Our review was originally published in 2008, and we have updated it to ensure that additional research is included so that the conclusions remain contemporary. In this article, we outline the most important outcomes that are relevent to the timing of treatment.
Material and methods
We identified studies using the Cochrane Oral Health Group’s Trials Register (to April 17, 2013), the Cochrane Central Register of Controlled Trials (Cochrane Library 2013, Issue 3), MEDLINE via OVID (1946 to April 17, 2013), and EMBASE via OVID (1980 to April 17, 2013). Articles that were identified as part of the Cochrane Oral Health Group’s hand searching program were obtained from the following journals: American Journal of Orthodontics and Dentofacial Orthopedics , Angle Orthodontist , European Journal of Orthodontics , Journal of Orthodontics , and British Journal of Orthodontics . An example of the search strategy is shown in the Appendix. We included randomized controlled trials that looked at children or adolescents, or both, receiving orthodontic treatment to correct prominent maxillary front teeth. The participants had to be 16 years of age or younger. We excluded trials that included participants with cleft lip or palate or other craniofacial deformities or syndromes. No language restrictions were placed on the studies considered for inclusion in this review, and published or unpublished sources were considered. For unpublished studies, an attempt was made to identify them by contacting the first-named author of the trial reports. The study eligibility was assessed by 2 review authors (B.T. and K.O’B.) independently and in duplicate, and disagreements were resolved by discussions or clarifications from the authors. Further details of the methodology that we used are included in the original review.
For the purposes of this article, we reported on only the most clinically relevant subset of outcomes from the original review.
The primary outcome measure was the prominence of the maxillary front teeth (overjet).
The secondary outcome measures were the relationship between the maxillary and mandibular jaws (cephalometric measurements), self-esteem and patient satisfaction (Piers Harris questionnaire), and incidence of incisal trauma.
The risk of bias was evaluated according to the Cochrane Collaboration’s tool for assessing the risk of bias, as described in the Cochrane Handbook for Systematic Reviews of Interventions . This was assessed independently by 2 authors (B.T. and another) against the following key criteria.
- 1.
Sequence generation: This evaluation was based on examination of the method used to generate the allocation sequence: eg, computer-generated random numbers or random number tables.
- 2.
Allocation concealment: These are the methods of concealing the allocation sequence from those assigning participants to the intervention groups. Did they use sealed envelopes, or was this done remotely via an Internet site or telephone allocation? These steps are taken so that the operator in the study cannot influence the treatment allocation.
- 3.
Blinding of participants, personnel, and outcome assessors: This ensures that the participants, clinicians, and assessors are unaware of the intervention allocations. This is carried out to reduce the chances that the operators and the data handlers could influence the results of the study. In orthodontic studies, it is often difficult to conceal the treatment allocation from participants and clinicians, but it is usually possible to blind the outcome assessors for data collection and analysis.
- 4.
Incomplete outcome data: This is an assessment of the possible effects of missing data caused by attrition or exclusion from analysis: eg, postrandomization dropouts. If there are many dropouts in a treatment group, this can introduce bias. This may be an issue in orthodontic studies because of relatively high dropout rates as a result of the long duration of the studies.
- 5.
Selective outcome reporting: Selectively reporting outcomes: eg, chasing significance or not reporting harms. This is assessed to make sure that all data are reported, not just the outcomes that are considered significant to the investigators.
- 6.
Other sources of bias: Biases not covered elsewhere.
We summarized the overall risk of bias for each study as low, unclear, or high.
Statistical analysis
The statistical analysis was performed according to the statistical guidelines referenced in the Cochrane Handbook for Systematic Reviews of Interventions and facilitated by RevMan.
Mean differences and 95% confidence intervals (CIs) were calculated for continuous data. Dichotomous outcomes were expressed as odds ratios (ORs) together with 95% CIs. Any heterogeneity between trials was assessed with the Cochran test and the I statistic. A meta-analysis was performed on studies with similar comparisons that reported the same outcome measures. We would have used the random-effects models if there had been more than 3 studies in the meta-analysis and used fixed-effect models if there were only 2 or 3 studies. When relevant, we calculated the numbers needed to treat and the risk ratio (RR).
Results
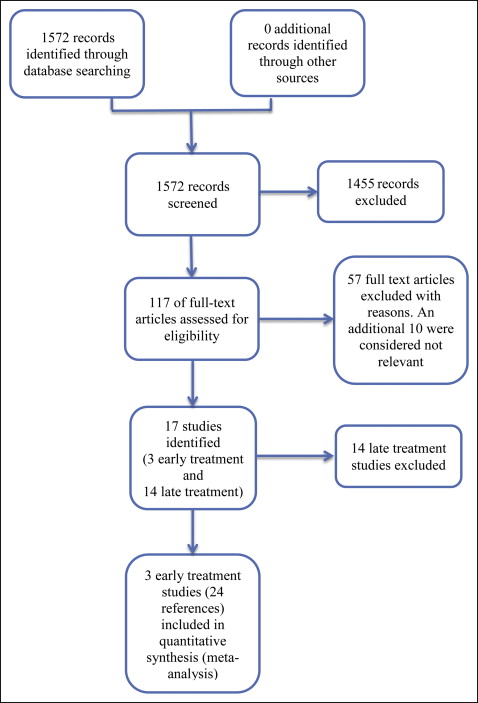
In our literature search, we initially identified a total of 1572 records, of which 117 full-text records were assessed. Of these, we excluded 57 articles; 10 additional studies were considered not relevant to this review. Seventeen trials were identified (published in 50 articles) ( Fig 1 ); these included 3 early treatment trials and 14 late treatment studies. The 3 early treatment trials are included in this review. These studies were reported in several articles, so for ease of description, we have combined them into 3 broad descriptors: Florida, North Carolina, and United Kingdom mixed.
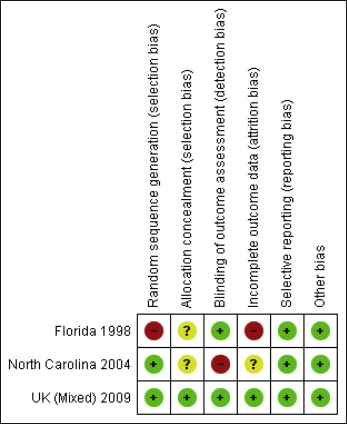
Assessment of the risk of bias revealed the following for each study.
In the Florida studies, there was a high risk of bias in 2 categories: bias in randomization method because the authors used a stratified randomization procedure, but after 3 years, the method was modified by allocation to groups (23% of the sample) because of the slow recruitment rate; and attrition bias, since the dropout rate was significantly higher for minority ethnic groups.
In the North Carolina studies, there was a high risk of bias for blinding of the outcome assessment. The molar bands were left in place at the end of phase 1 data collection, allowing the technician to identify these patients’ treatment groups.
In the United Kingdom mixed studies, there was a low risk of bias.
Further more detailed information on the risks of bias for each study is given in Table I and Figure 2 .
| Bias | Authors’ judgment | Support for judgment |
|---|---|---|
| Florida | ||
| Random sequence generation (selection bias) | High risk | A stratified block randomization procedure was used “Subjects initially were selected in blocks of 6 and randomized to the treatment protocols. This procedure of assigning subjects to groups only after a block had filled was modified in year 3, after we recognized slow entry rate and many partially filled blocks (23% of the sample) were randomized to groups” |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | Low risk | “All cephalometric radiographs were encoded by the staff assistant and then decoded for analysis” |
| Incomplete outcome data (attrition bias) | High risk | Clear information on withdrawals. Dropouts, 24%. Number of dropouts was approximately equal in each group, but the rate of withdrawal was significantly higher for subjects who were not white. |
| Selective reporting (reporting bias) | Low risk | All variables reported |
| Other biases | Low risk | No other sources of bias identified |
| North Carolina | ||
| Random sequence generation (selection bias) | Low risk | “Randomization was performed within gender in blocks of six patients with Proc Plan in SAS” |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) | High risk | Because the molar bands were not removed at the end of phase 1, the technician was not masked as to these patients’ treatment group |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of patients randomized in different groups not reported 192 randomized; 175 started, 166 finished phase 1; and 137 finished phase 2 Dropout rates of 13.5% (low risk) for phase 1 and 28.6% (high risk) for phase 2. Reasons for dropouts reported, but not for each treatment group |
| Selective reporting (reporting bias) | Low risk | All variables reported |
| Other biases | Low risk | No other bias found |
| United Kingdom mixed | ||
| Random sequence generation (selection bias) | Low risk | “The randomization was made at the start of the study with pre-prepared random number tables with a block stratification on centre and sex” |
| Allocation concealment (selection bias) | Low risk | Randomization was carried out by using a central telephone line and minimization software |
| Blinding of outcome assessment (detection bias) | Low risk | Assessor blinded to outcomes. “The cephalograms and the study casts were scored with the examiner unaware of the patient’s group” |
| Incomplete outcome data (attrition bias) | Low risk | Clear information on withdrawals, but rates different in each group: 22/89 (25%) in the Twin-block group and 12/85 (14%) in the control group. Reasons for exclusion specified (unpublished data) Control group: 4 refused to consent to phase 2 treatment, 1 withdrew due to illness, 3 had multiple DNAs with no final records, 1 moved away or lost contact, 2 had Twin-blocks fitted in phase 1 in error, 1 had a sore mouth and required treatment in phase 1 Treatment group: 2 moved away or lost contact, 9 had multiple DNAs with no follow-up records, 4 did not start because eligibility criteria were not met, 5 refused to continue, 1 had poor oral health, 1 was removed from study because of health problems |
| Selective reporting (reporting bias) | Low risk | All variables reported |
| Other biases | Low risk | Groups appeared similar at baseline |
Three trials reported on early treatment for Class II Division 1 malocclusion, and all were included in the meta-analyses. Detailed characteristics are described in Table II .
| Study | Characteristics |
|---|---|
| Florida | |
| Methods | Location: University of Florida Number of centers: 1 Recruitment period: not stated Funding source: funded by NIH (DE08715) Trial design: randomized parallel group study over 10 years |
| Participants | Inclusion criteria: third or fourth grade at school, at least bilateral 1/2 cusp Class II molars or 1 side <1/2 cusp Class II if other side was greater than 1/2 cusp Class II. Fully erupted permanent first molars, emergence of not more than 3 permanent canines or premolars, and positive overbite and overjet Exclusion criteria: not willing to undergo orthodontic treatment or to be randomly allocated to treatment type. Poor general health, active dental or periodontal pathology Age at baseline: mean, 9.6 years Screened child population (360) then referred to clinic for treatment Number randomized: 325 randomized; 277 started treatment: 95, 100, and 82 in bionator, headgear, and control groups, respectively Number evaluated: end of treatment phase (1), 79/95, 92/100, and 78/82; end of retention phase, 75/95, 85/100, and 75/82; and end of follow-up (II), 70/95, 81/100, and 74/82 in bionator, headgear, and control groups, respectively |
| Interventions | Group A: bionator appliance Group B: cervical pull headgear with removable biteplane Group C: delayed treatment control 3 phases of treatment: 2 years of early treatment plus 6 months retention plus further 6 months follow-up |
| Outcomes | Overjet Skeletal discrepancy Dental alignment measured with the PAR index |
| Notes | Duration of randomized treatment: 2 years initially Sample size calculation not reported |
| North Carolina | |
| Methods | Location: North Carolina Number of centers: 1 Recruitment period: August 1988 to November 1993 Funding source: grants from NIH, and Orthodontic Fund, Dental Foundation of North Carolina Trial design: parallel group randomized controlled trial with 2 treatment phases |
| Participants | Inclusion criteria: children with mixed dentition, with all permanent teeth developing, with growth potential throughout phase 1 of treatment. Overjet >7 mm, all incisors erupted, second molars not erupted Exclusion criteria: clinically obvious facial asymmetry, cleft or syndrome, more than 2 SD from normal vertical proportionality, and prior orthodontic treatment Age group: mean, 9.4 years (SD, 1.0 year) Screened child population (2164) then referred to clinic for treatment Numbers randomized: 192 randomized, 175 started treatment Numbers evaluated: 53, 52, and 61 at the end of phase 1, and 39, 47, and 51 at the end of phase 2 for bionator, headgear, and control groups, respectively |
| Interventions | Group A (n = 53): functional appliance—modified bionator with the bite taken with 4-6 mm of protrusion and minimal vertical opening. Reactivation of appliance when necessary was by construction of a new appliance Group B (n = 52): headgear—combination headgear with supershort outer bow, adjusted to deliver 8-10 oz to the head cap, with neck strap force just sufficient to prevent buccal flaring of maxillary molars All appliances delivered within 1 month of patients’ initial records being taken Group C (n = 61): control (observation only) |
| Outcomes | Skeletal growth changes; maxilla, mandible, skeletal relationship, dental relationship |
| Notes | Duration of intervention: phase 1, 15 months, and phase 2, 25.5, 30.1, and 34.5 months for functional, headgear, and control groups, respectively Frequency of treatment visits: every 6-8 weeks for active treatment groups and every 6 months for control group Sample size calculation: sample size of 40 per group was calculated as necessary to detect a mean difference between any 2 groups equivalent to the doubling in annualized change of SNPg (with alpha = 0.01 and power of 0.90) Patients were rerandomized at the end of phase 2 for different clinicians |
| United Kingdom | |
| Methods | Location: United Kingdom Number of centers: 13 Recruitment period: March 1997 to June 1998. Funding source: Medical Research Council (99410454) Trial design: randomized parallel group trial |
| Participants | Inclusion criteria: children in the mixed dentition with overjet greater than 7 mm and willingness of the patient and a parent to participate in the study. The patients had to be in the mixed dentition with at least the permanent incisors and first molars erupted, but there was no age criterion Exclusion criteria: craniofacial syndromes Age at baseline: average ages were 9.7 (SD, 0.98) years for the treatment group and 9.8 (SD 0.94) years for the control group Number randomized: 174 Number evaluated: 127 |
| Interventions | Comparison Group A: Twin-block early treatment: randomized, 89; completed, 67 Group B: Twin-block delayed treatment: randomized, 85; completed, 73 |
| Outcomes (trauma not noted) | Overjet Skeletal discrepancy measured by the Pancherz analysis Dental alignment measured with the PAR index Duration of treatment |
| Notes | Duration of intervention: phase 1, 15 months; phase 2, early treatment group, 14 months (435 days), late treatment group, 24 months (744 days). Sample size calculation: “This showed that the mean duration of treatment for patients who had later treatment after early treatment was 25 months (SD, 11). It was decided that a meaningful difference between the treatment duration for children who did, or did not, receive early treatment was 6 months. To give a study a power of 80% and an alpha of 0.05, the sample size needed to be 60 in each group” |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


