Introduction
There are multiple causes of external root resorption, but absent a disease state, it is most often observed when excessive physical force is used during orthodontic treatment. Even without mechanical stimulation, however, root resorption can still occur. The purpose of this study was to test whether Wnt signaling plays a role in pathologic root resorption, by conditionally deleting Wntless (Wls) from odontoblasts and osteoblasts and then evaluating the phenotypic effects on the maintenance of the root surface.
Methods
Ten (age, 1 month) and 20 (age, 3 months) OCN-Cre;Wls fl/fl mice and their wild-type littermates were evaluated using microcomputed tomography, histology, and immunohistochemistry. Phenotypic alterations in the alveolar bone, dentin, and cementum were characterized and quantified.
Results
In a genetic model of reduced Wnt signaling, we found that RANKL expression is upregulated, and osteoprotegerin expression is downregulated. This molecular disruption results in an increase in osteoclast activity, a decrease in osteoblast activity, and extensive, spontaneous root resorption. A genetic strain of mice in which Wnt signaling is elevated exhibits thicker cementum, whereas, even in the perinatal period, OCN-Cre;Wls fl/fl mice exhibit thinner cementum.
Conclusions
Taken together, these data demonstrate that Wnts regulate cementum homeostasis, and that idiopathic cases of root resorption might have as their etiology a reduction in endogenous Wnt signaling.
Highlights
- •
Wnts regulate cementum homeostasis.
- •
Idiopathic root resorption might be related to reduction in endogenous Wnt signaling.
Root resorption is defined as the pathologic removal of cementum and dentin, and its etiology has perplexed dentists since first being described in 1948. This resorptive process can occur in response to imbalances in the endocrine system, although this is rare; more commonly, root resorption is associated with the use of excessive force or an extended duration of orthodontic tooth movement. The extent of apical displacement, the method of force application, and various patient-related factors are also implicated in the etiology of root resorption. Because there is only a cursory understanding of the factors (physical, chemical, biologic) responsible for maintaining the integrity of the cementum, there is little to guide clinicians on how to prevent or limit root resorption once it is detected.
Some clues into the biologic regulation of cementum formation (and therefore root resorption) have come from studies focusing on the activity of the osteoprotegerin (OPG)/receptor activator of nuclear factor kappa B ligand (RANKL) pathway. OPG encodes a soluble receptor that blocks the activity of RANKL. OPG/RANKL regulates bone formation, and more studies in the literature suggest that disruptions in OPG/RANKL contribute to low bone mass phenotypes in humans and perhaps root resorption as well. The OPG/RANKL pathway, in turn, is mediated by Wnt signaling: a positive Wnt stimulus simultaneously downregulates RANKL and upregulates OPG, thus enhancing bone formation and inhibiting bone resorption.
Here, we directly tested the hypothesis that Wnt signaling mediates root resorption via its regulation of OPG and RANKL. We used a Cre-LoxP system to conditionally delete Wntless (Wls), a chaperone protein that escorts lipid-modified Wnts from the Golgi to the cell surface. To block Wnt signaling specifically in mineralized tissues, Wls fl/fl mice were crossed with osteocalcin (OCN) Cre mice to generate OCN-Cre;Wls fl/fl offspring. A Wnt-deficient environment was thus created in the periodontal complex; this allowed us to assess the function of this pathway in cementum maintenance and, conversely, root resorption.
Material and methods
OCN-Cre;Wls fl/fl mice were generated as described with approval by the Van Andel Research Institute Institutional Animal Care and Use Committee (IACUC) (protocol number 13-03-015). Ten (age, 1 month) and 20 (age, 3 months) OCN-Cre;Wls fl/fl mice and their wild-type littermates were analyzed.
The generation of Axin2 LacZ/+ mice was performed as described with approval by Stanford University IACUC (protocol number 13146). Ten 2-month-old mice were used in this study. Cells responsive to Wnt signaling express the LacZ gene product, beta-galactosidase, which is detected by X-gal staining. To perform X-gal staining, tissues were fixed in 0.4% paraformaldehyde overnight before being decalcified with 19% EDTA and infused with 30% sucrose for 24 hours. Samples were embedded in optimum cutting temperature medium and cryosectioned at a thickness of 8 μm. Tissues were then fixed in 0.2% gluteraldehyde for 15 minutes and stained with X-gal overnight at 37°C.
Microcomputed tomography analyses (5 wild-type, 5 OCN-Cre;Wls fl/fl ) were taken using MicroXCT-200 (SkyScan, Kontich, Belgium) at 60 kV and 7.98 W with a resolution of 2 μm. Scans were acquired using an 8-μm isotropic voxel size, with 800 computed tomography slices evaluated in the incisor area. For analyses, individual computed tomography slices were reconstructed with MicroXCT reconstruction software (version 7.0; SkyScan), and data were analyzed with Inveon Research Workplace (Erlangen, Germany).
Samples including the teeth and surrounding tissues were sectioned in a transverse plane, and the analysis for cementum thickness was done using 3 serial sections from the midbuccolingual area. Measurements were taken at 3 defined locations corresponding to midpoint and at levels 80 μm coronal and apical to the midpoint on both the mesial and distal sides of the tooth.
Maxillae from the 1-month-old mice (5 wild-type, 5 OCN-Cre;Wls fl/fl ) and 3-month-old mice (10 wild-type, 10 OCN-Cre;Wls fl/fl ) were harvested and fixed in 4% paraformaldehyde overnight at 4°C. The samples were decalcified in a heat-controlled microwave in 19% EDTA for 2 weeks. After demineralization, the specimens were dehydrated through an ascending ethanol series before they were embedded in paraffin. Longitudinal sections (thickness, 8 μm) were cut and collected on slides for histology.
Tissue sections were deparaffinized according to standard procedures. Relevant digoxigenin-labeled mRNA antisense probes were prepared from complementary DNA templates for OCN. Sections were deparaffinized, treated with proteinase K, and incubated in hybridization buffer containing the relevant RNA probe. The probe was added at an approximate concentration of 1 μg per milliliter. Stringency washes of saline sodium citrate solution were done at 65°C and further washed in maleic acid buffer with 1% Tween 20 (Roche Diagnostics, Indianapolis, Ind). Slides were treated with an antibody to Anti-digoxigenin-AP (Roche Diagnostics). For color detection, the slides were incubated in nitro blue tetrazolium chloride (Roche Diagnostics) and 5-bromo-4-chloro-3-indolyl phosphate (Roche Diagnostics). After developing, the slides were coverslipped with permount mounting medium.
Movat’s pentachrome staining was performed. Nuclei stain blue to black, cytoplasm stains red, collagen stains yellow to greenish yellow, and fibrous tissue stains an intense red. Tissues were also stained with the acidic dye, picrosirius red, to discriminate tightly packed and aligned collagen molecules. Under polarized light, well-aligned fibrillary collagen molecules present polarization colors of longer wavelengths (red) compared with less organized collagen fibrils that show colors of shorter wavelengths (green-yellow).
For alkaline phosphatase staining, the slides were preincubated overnight at 4°C in alkaline phosphatase buffer containing 100 mmol/L Tris (pH 9.5), 50 mmol/L magnesium chloride, 100 mmol/L sodium chloride, and 0.1% Tween 20. Slides were incubated in BM-purple solution (Roche Diagnostics) overnight at 4°C until a dark purple color reaction appeared.
For the immunohistochemistry, tissue sections were deparaffinized according to standard procedures. Endogenous peroxidase activity was quenched by 3% hydrogen peroxide for 5 minutes and then washed in phosphate-buffered saline (PBS). Slides were blocked with 5% goat serum (Vector S-1000; Vector Labs, Burlingame, Calif) for 1 hour at room temperature. The appropriate primary antibody was added and incubated overnight at 4°C and then washed in PBS. Samples were incubated with appropriate biotinylated secondary antibodies (Vector BA-x) for 30 minutes and washed in PBS. An advidin/biotinylated enzyme complex (Kit ABC Peroxidase Standard Vectastain PK-4000) was added and incubated for 30 minutes, and a DAB substrate kit (Kit Vector Peroxidase subtrate DAB SK-4100) was used to develop the color reaction. Antibodies used include dentin sialoprotein (dilution 1:2000; Merck KGaA, Darmstadt, Germany), osteopontin (LF-175, dilution 1:4000; NIH, Bethesda, Md), OPG (dilution 1:200; Biorbyt, San Francisco, Calif), and RANKL (dilution1:400; Novus, Littleton, Colo).
For the measurement of tartrate-resistant acid phosphatase (TRAP) activity, approximately 40 slides were generated from sectioning each tissue sample, and 6 to 8 tissue sections were used for analysis. Each section was photographed using a digital imaging system (Leica, Wetzlar, Germany). The digital images were imported into Adobe Photoshop CS2 (Adobe Systems, San Jose, Calif). The region of interest typically encompassed 106 pixels. The number of TRAP–stained pixels was determined using the Magic Wand tool (tolerance setting: 50; histogram pixel setting: cache level 1) by 1 blinded investigator (D.C.) and confirmed by a second independent investigator (S.M.). These data then were collected from the first, second, and third molar roots.
Statistical analysis
The results are presented as the means ± standard deviations. The Student t test was used to quantify differences described in this article. One asterisk denotes a P value of <0.05, and 2 asterisks denote a P value of <0.01.
Results
Loss of Wnt secretion causes root resorption
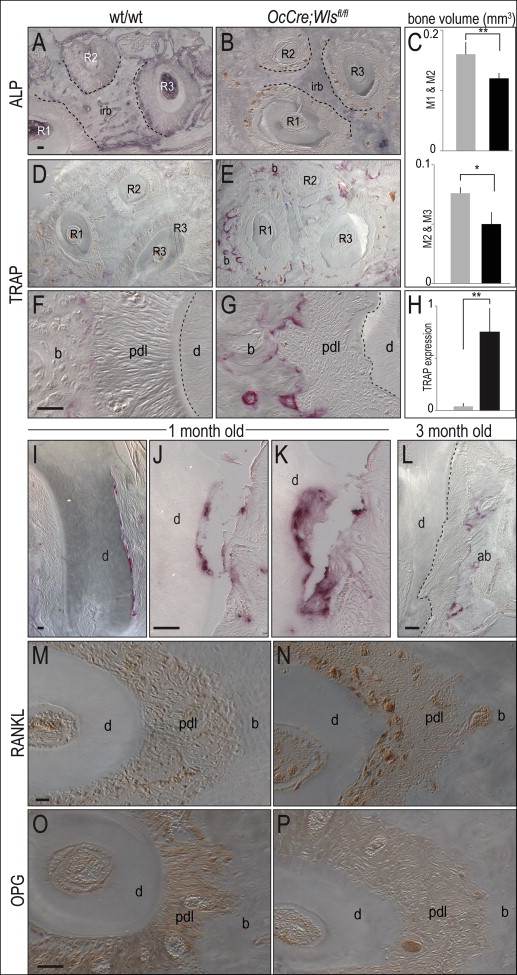
Molars in OCN-Cre;Wls fl/fl mice erupt normally, and the gross morphology and appearance of the mutant crowns appeared equivalent to wild-type molars ( Fig 1 , A-C ). The root surfaces of the OCN-Cre;Wls fl/fl teeth, however, were noticeably irregular: 3-dimensional isosurface reconstruction of the microcomputed tomography data showed extensive cratering, most notably at the cementoenamel junction (compare Fig 1 , D and E with F and G ). Histologic examination confirmed extensive root resorption in OCN-Cre;Wls fl/fl mice, which showed that the cementum was eroded and the dentin was penetrated at multiple locations along the molar root surfaces ( Fig 1 , H-K ).
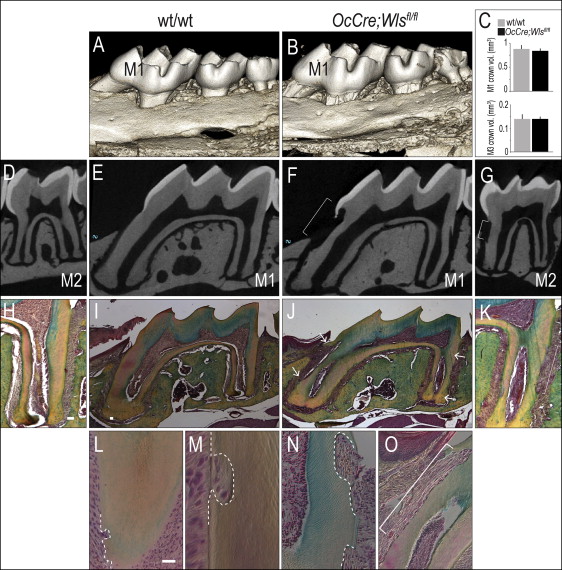
Closer examination showed resorptive lesions, ranging from small (20-50 μm in diameter), focal defects near the root apices of the 1-month-old OCN-Cre;Wls fl/fl mice ( Fig 1 , L ), to moderate-sized (50-100 μm) defects ( Fig 1 , M ). There were also larger (>100 μm) cavitating lesions that left little of the cementum intact in the 3-month-old OCN-Cre;Wls fl/fl mice ( Fig 1 , N and O ).
Loss of Wls results in defective cementum and root dentin
The mineral density and ultrastructure of bone are severely impacted by the loss of Wnt signaling; in a previous report, we characterized the alveolar bone defect in OCN-Cre;Wls fl/fl mice. The similarities between bone and cementum and the spontaneous root resorption we observed ( Fig 1 ) prompted us to more closely examine the cementum and dentin of the OCN-Cre;Wls fl/fl mice for abnormalities in matrix deposition.
Compared with wild-type dentin ( Fig 2 , A ), the expression of dentin sialoprotein was higher in OCN-Cre;Wls fl/fl odontoblasts ( Fig 2 , B ). Osteopontin protein expression, on the other hand, was reduced in the cementum of the OCN-Cre;Wls fl/fl mice ( Fig 2 , C and D ). Additional matrix molecules including dentin phosphoprotein, OCN, alkaline phosphatase, Runx2, and osterix showed no changes between the mutant and the wild-type mice (data not shown).
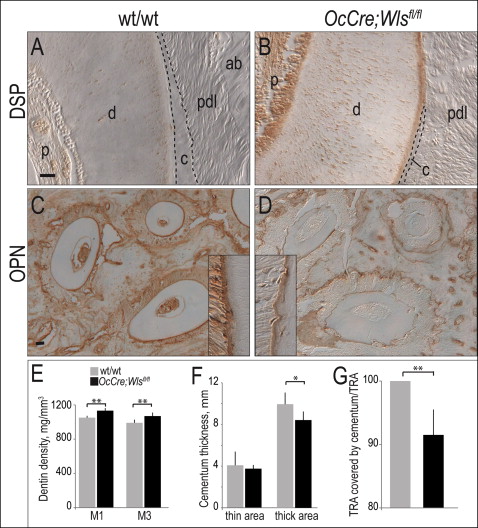
These subtle shifts in the expression levels of a few matrix components were accompanied by a physical change in mineral density: microcomputed tomography analyses demonstrated that in OCN-Cre;Wls fl/fl mice the dentin was denser ( Fig 2 , E ) and the cementum was thinner than in the wild-type littermates ( Fig 2 , F and G ).
Wnt signaling regulates cementum thickness
There were 2 possible explanations for the spontaneous root resorption we observed in the OCN-Cre;Wls fl/fl mice: (1) it was possible that the cementum failed to attach properly to the dentin and thus was lost, and (2) the cementum, like the alveolar bone, was thinner because of excessive resorption. To distinguish between these 2 possibilities, we first pinpointed the cells in the periodontal ligament space that were defective in Wnt signaling in this conditional mutant.
In the OCN-Cre;Wls fl/fl mice, the Wls gene is specifically removed in cells expressing OCN. Since the root resorption phenotype was only observed after birth, we concentrated on mapping the expression of OCN in 1-month-old and 3-month-old mice. In the wild-type mice, OCN expression is strongly expressed in osteoblasts ( Fig 3 , A ) and in OCN-Cre;Wls fl/+ mice; cells lining the alveolar bone were also positive for OCN expression ( Fig 3 , B ) but were sparse compared with their distributions in the wild-type mice ( Fig 3 , C ). Neither the wild-type nor the mutant cementoblasts expressed OCN ( Fig 3 , C ). Together, these analyses indicated that reduced Wnt signaling in the periodontal complex caused root resorption. We sought support for this hypothesis by examining the periodontal complex in mice with an elevated level of Wnt signaling.
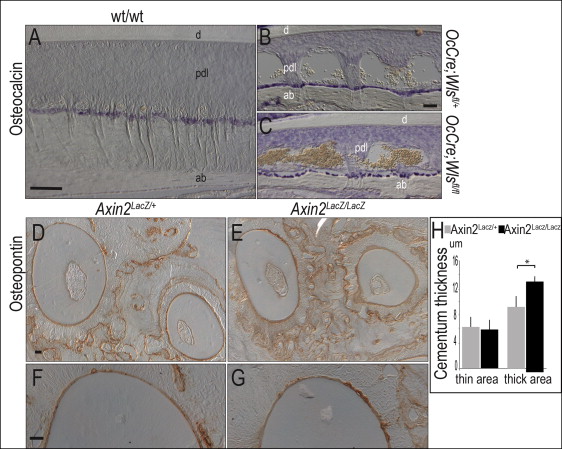
Mice carrying a genetic deletion in Axin2, a negative regulator of Wnt signaling, exhibit a ligand-dependent elevation in Wnt signaling. Using microcomputed tomographic, histologic, and molecular analyses, we found no differences in dentin thickness or density of Axin2 LacZ/LacZ mice compared with heterozygous Axin2 LacZ/+ and wild-type littermates (data not shown). Likewise, cellular analyses using osteopontin ( Fig 3 , D-G ), dentin sialoprotein, and dentin phosphoprotein (not shown) showed no obvious differences in dentin appearance.
An elevated Wnt signaling environment is associated with increased cementum formation. For example, in Axin2 LacZ/LacZ mice, the cementum was measurably thicker than that observed in the heterozygous littermates ( Fig 3 , H ). If cementum thickness is under Wnt control, then root resorption in OCN-Cre;Wls fl/fl mice might be due to a defect in cementum formation. Alternatively, the phenotype might arise because the cementum is being eroded at a higher-than-normal rate. To clarify these possibilities, we examined mutant and control mice for variations in osteoblast, cementoblast, and osteoclast activities in the periodontal space.
Osteoblasts undergoing mineralization exhibit robust alkaline phosphatase activity ; compared with wild-type mice, this activity was significantly reduced in the periodontal complex of OCN-Cre;Wls fl/fl mice ( Fig 4 , A and B ). The volume of interradicular bone was also significantly decreased ( Fig 4 , C ).