Introduction
The selection of appropriate outcomes is a fundamental part of the design of clinical trials. Orthodontic treatment aims to improve a person’s dentofacial appearance, and research outcomes should therefore reflect the perspectives of both clinicians and patients. In this study, we aimed to identify which outcomes were measured in recent orthodontic trials and to explore whether any relevant outcome domains were underrepresented.
Methods
Five electronic databases were searched to identify all randomized controlled trials of orthodontic treatment interventions in children published in the last 5 years. Abstracts and eligible full-text articles were screened independently and in duplicate by 2 reviewers. Outcome measures were identified and categorized into 6 predetermined outcome domains.
Results
The search identified 650 abstracts, of which 244 eligible articles were retrieved in full. One hundred thirty-three studies met the inclusion criteria and were included. Morphologic features of malocclusion were measured in 84 studies (63%); health resource utilization in 43 (32%); adverse effects of orthodontic treatment in 43 (32%); quality of life in 12 (9%); functional status in 10 (8%); and physical consequences of malocclusion in 3 (2%). There was no consistency in the outcomes selected among the trials to measure these domains.
Conclusions
Most of the outcomes used in orthodontic research are concerned with measuring morphologic changes of treatment and do not reflect patient perspectives. Five of the 6 domains were infrequently evaluated, and outcomes were heterogenous. A core set of outcomes for clinical trials of orthodontic treatment interventions would help to overcome these issues.
In this article, we intend to outline a study in which we evaluated whether outcome measures that have been used in orthodontic trials since 2008 are relevant to patients.
First, when considering the factors that should influence the selection of outcome measures for orthodontic research, a useful starting point is to consider the nature of malocclusion. Malocclusion is not a disease but, rather, a variation from an accepted societal norm that can lead to functional difficulties or concerns about dentofacial appearance for a patient. As a result, malocclusion falls under the World Health Organization’s framework of functioning, disability, and health, which considers the psychologic and sociologic in addition to the purely biologic aspects of disability. Therefore, it can be suggested that malocclusion might be a chronic disability that is amenable to treatment that can render a patient back to a state of oral health.
There have been many definitions of oral health; arguably, the most accepted is that proposed by Dolan, as “a comfortable and functional dentition which allows individuals to continue in their desired social role.” Importantly, this takes into account the social and functional elements as fundamental aspects. It is implied that when we study the treatment of malocclusion, within the broader context of oral health, the measurement of perceptions and behaviors is as essential as the measurement of the “disability” itself. Hence, research outcomes should reflect this.
Although the adoption of randomized clinical trial (RCT) methodology in orthodontic research is increasing, it has been suggested that the reported outcomes appear to be mostly relevant to clinicians and not to our patients. This is important because the relevance of a study is derived from the outcomes it reports, so concentrating on measures that are important only to clinicians might fail to consider pertinent issues. This has been succinctly stated by Sinha et al, who evaluated the outcomes used in clinical trials for the treatment of childhood asthma and reported that “the selection of inappropriate outcomes can lead to wasted resources or misleading information that overestimates, underestimates, or completely misses the potential benefits of an intervention.” The issues arising from poor outcome measurement and reporting in studies are therefore multifaceted. First, the time and resources invested in the research are wasted, with approximately 40% to 89% of published trials not replicable due to poor descriptions of their interventions and outcomes. Second, valuable information regarding the effectiveness of an intervention can be overlooked by neglecting to measure outcomes important to patients. As a result, including patient values is at the core of evidence-based medicine, and integration of these values with clinical research evidence is necessary to enable decision making.
Another frequently encountered problem is the difficulty in combining the results of trials into systematic reviews, because the selection of outcome measures in the trials has been inconsistent. This outcome heterogeneity was clearly demonstrated in the Cochrane review on the treatment of increased overjets, where the included studies all used different cephalometric analyses to answer the same questions.
Recently, there has been extensive work in the initial stages of development of an agreed standardized set of outcomes for health care. These are termed core outcome sets (COS). It is suggested that “these outcomes should be measured as a minimum in trials assessing effectiveness of interventions, and would help eliminate issues relating to outcome heterogeneity and outcome reporting bias while ensuring that the perspectives of both clinicians and patients are measured, thus enhancing the value of RCTs and systematic reviews.” At present, COS development in dentistry and in certain fields of medicine is still in its infancy. However, in others and most notably in rheumatology, such work has advanced greatly through the work by the OMERACT initiative. This international collaboration used standardized consensus techniques to develop COS in clinical trials of rheumatology to reduce the discrepancies and inconsistencies in outcome measurement that mainly existed between United States and Europe. Currently, there are no COS available for orthodontic trials.
The aims of this study were to (1) identify the outcomes measured in recent orthodontic trials, (2) classify them into various outcome domains, (3) consider whether they were relevant to patients, and (4) suggest whether any relevant outcome domains were underrepresented.
Material and methods
The outcomes used in previous orthodontic research were evaluated by conducting a systematic review of the literature. Studies were considered eligible if they met the following inclusion criteria.
- 1.
Study design: prospective RCTs. All parallel-group RCTs, including those of crossover or cluster design, were considered eligible for inclusion.
- 2.
Participants: children up to age 16 years at the start of treatment, including those with nonsyndromic clefts, were eligible.
- 3.
Interventions: any orthodontic treatment intervention with no restrictions placed on the control groups was eligible.
- 4.
Outcome measures: all reported outcomes (primary and secondary) were to be identified.
- 5.
Exclusions: studies involving solely adults, patients with syndromic conditions, surgical or pharmacologic interventions, and purely laboratory investigations were excluded.
The electronic search strategy was designed to include the relevant literature, published from January 1, 2008, through December 31, 2012. The following electronic databases were searched: MEDLINE via Ovid, EMBASE via Ovid, the Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCO, and the Cochrane Central Register of Controlled Trials (CENTRAL, Cochrane Library). The search strategy was informed by the identified PICO concepts and inclusion criteria, and was tailored to each database to ensure appropriate use of search terms and limits. Controlled vocabulary using appropriate subject headings (MeSH terms), as well as free text search terms, were used as necessary, before the identified terms and concepts were grouped together with Boolean operators. No language restrictions were applied.
In addition, the reference lists and trials identified in recently published Cochrane systematic reviews were crosschecked to ensure that no relevant studies were missed from the electronic search.
The abstracts of all studies identified by the searches were assessed independently and in duplicate by 2 reviewers (A.T. and K.O’B.). Full-text reports of studies that appeared to meet the inclusion criteria and of studies for which there was insufficient information in the title or abstract to make a clear decision were obtained. The 2 reviewers independently assessed the full-text articles, and any disagreement regarding final inclusion was resolved by discussion until a consensus was reached.
The primary and any secondary outcomes were identified from the information stated by the authors. If this was not clear, the primary outcome was inferred from the aim of the study and any sample size calculation. Any subsequent outcomes reported in the results were also identified and recorded in a previously piloted data extraction spreadsheet as secondary outcomes. In the event of uncertainty as to which outcomes constituted the primary and secondary outcomes, all were recorded as primary outcomes, and a note of this was made in the data extraction sheet.
The reviewers independently categorized the outcomes into the following domains using the method adopted by Sinha et al : (1) disease activity (morphologic features or changes of malocclusion), (2) physical consequence of malocclusion, (3) functional status, (4) social outcomes and quality of life, (5) health service resource utilization, and (6) adverse effects of treatment.
For the purpose of this review, there was no synthesis of outcome data; therefore, an overall methodologic quality appraisal of the studies was not necessary because the outcomes might not be reflected by the quality of the trials.
Results
We identified 650 articles through the searches, after removing duplicates. After screening, we considered that 244 abstracts were eligible for inclusion, and full-text articles were sought. After careful examination of the remaining full texts, 133 studies met the inclusion criteria and were included in the review. The PRISMA flow diagram is shown in Figure 1 .
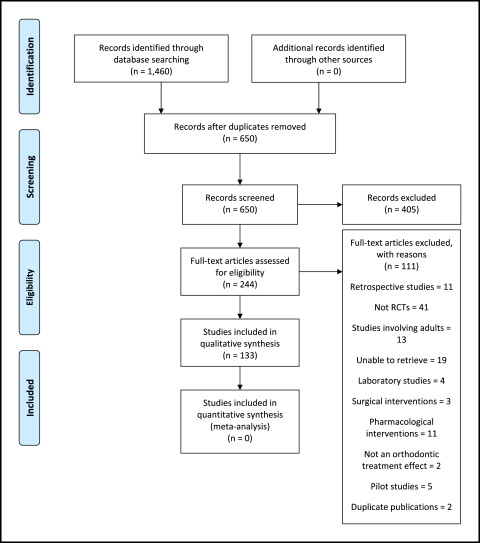
When we found publications derived from the same trial but involving different outcome measures or different follow-up periods, we considered these to be separate studies. We did not find any RCTs on the treatment of children with clefts published during this period.
Most studies were carried out in single centers (n = 104, 78%), of which 23 (17%) used a split-mouth design. Twenty-one studies (16%) were multicentered, whereas for 8 (6%) the number of centers involved was unclear.
A sample size calculation was reported in half (n = 65, 49%) of the studies. In 5 studies (4%), it was not clear whether a sample size calculation had been performed. In 6 (5%) of those that reported a sample size calculation, the primary outcome on which this was based was not clearly stated. In 2 studies (2%), the outcome used for the sample size calculation was different from the authors’ stated primary outcome. In these cases, both outcomes were noted as primary outcomes during data extraction.
Table I includes data on the identified outcomes and domains. This shows a wide variety of outcomes reported across the studies, even within the same domains.
| Domain | Subdomain | Outcome | Number (%) of studies that measured the outcome reported as any outcome | Number (%) of studies that measured the outcome reported as the primary outcome |
|---|---|---|---|---|
| Disease activity (features of malocclusion) | Study cast measurements | Tooth movement (mm and °) | 17 (13) | 7 (2) |
| Dental alignment (Little’s index/HLD index) | 12 (9) | 12 (9) | ||
| Arch-width changes | 15 (11) | 9 (7) | ||
| Occlusal outcome (PAR) | 12 (9) | 8 (6) | ||
| Occlusal outcome (ICON) | 2 (2) | 0 (0) | ||
| Bite registrations | Areas of contact and near contact | 2 (2) | 2 (2) | |
| Cephalometric measurements | Dental changes (mm and °) | 33 (25) | 25 (19) | |
| Skeletal changes (mm and °) | 24 (18) | 15 (11) | ||
| Soft-tissue changes (mm and °) | 9 (7) | 8 (6) | ||
| DPT measurements | Dentoalveolar changes | 3 (2) | 2 (2) | |
| CBCT/CT measurements | Dentoalveolar changes | 5 (4) | 5 (4) | |
| Ultrasound measurements | Skeletal (condylar) changes | 1 (1) | 1 (1) | |
| Clinical (intraoral) findings | Eruption of teeth | 4 (3) | 3 (2) | |
| Periodontal condition (BPE/GCF volume) | 2 (2) | 1 (1) | ||
| AC of IOTN | 1 (1) | 0 (0) | ||
| Dental alignment (Little’s index) | 1 (1) | 0 (0) | ||
| Notes/records review | Dental changes (overjet/crossbite correction) | 3 (2) | 2 (2) | |
| Adverse effects of treatment | Radiographic findings (CT, PA) | Root resorption | 5 (4) | 4 (3) |
| Laboratory findings | Plaque/bacterial accumulation | 4 (3) | 4 (3) | |
| Clinical findings | Plaque accumulation/periodontal condition | 5 (4) | 3 (2) | |
| Demineralization | 4 (3) | 2 (2) | ||
| Complications | 4 (3) | 0 (0) | ||
| Questionnaire | Pain/discomfort | 22 (17) | 11 (8) | |
| Patient expectations of treatment | 5 (4) | 3 (2) | ||
| Health resources utilization | Clinical findings | Attachment/appliance failure | 22 (17) | 16 (12) |
| Treatment duration | 19 (14) | 10 (8) | ||
| Cost analysis | 2 (2) | 0 (0) | ||
| Patient motivation and/or compliance | 2 (2) | 1 (1) | ||
| Questionnaire | Patient motivation and/or compliance | 2 (2) | 0 (0) | |
| Questionnaire | Clinician’s preference | 2 (2) | 1 (1) | |
| Quality of life | Questionnaire | Patient perceptions of occlusion/impact of malocclusion | 5 (4) | 0 (0) |
| Patent acceptability of treatment | 3 (2) | 3 (2) | ||
| Patient anxiety | 2 (2) | 1 (1) | ||
| Interview | Patient perceptions of occlusion | 1 (1) | 0 (0) | |
| Functional status | Clinical findings | Intraoral examination (TMJ) | 3 (2) | 1 (1) |
| Self-reported questionnaire | TMJ/jaw impairment | 2 (2) | 1 (1) | |
| CT measurements | Condylar position | 1 (1) | 1 (1) | |
| Rastereography | Back/spine position | 1 (1) | 1 (1) | |
| Inclinometer | Head posture | 1 (1) | 1 (1) | |
| EMG | Muscle activity | 2 (2) | 2 (2) | |
| Physical consequence of malocclusion | Clinical findings | Incisor injury | 2 (2) | 1 (1) |
| Relapse | 2 (2) | 1 (1) |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


