Fig. 4.1
Basic steps in the diagnosis of dentin hypersensitivity
Holland and coworkers (1997) described dentin hypersensitivity as a condition that manifests as a brief and acute pain that cannot be attributed to any other form of dental pathology or defect. Dentin hypersensitivity is a painful clinical condition which requires knowledge of the neuroanatomy of the pulp dentin complex, the physiology of pain and methods of interpreting it to reach a correct diagnosis.
4.2 Innervation of Human Dentin
The dental pulp is a highly innervated organ (Trowbridge 1985; Ten Cate 1994). Nerves enter the pulp through the apical foramen with afferent blood vessels and form neurovascular bundles. They traverse the root canal until they reach the pulp chamber, where the nerve fibers commence to divide and send branches to the surrounding coronal dentin. The nerves form an interlacing network as they approach the subodontoblastic region, known as the subodontoblastic plexus of Raschkow. Here, the myelinated fibers lose their myelin and become free nerve endings. It is estimated that as each nerve fiber enters the pulp, it branches into eight terminal fibers at the pulp-dentin interface. Gunji (1982) classified the nerve endings of these branches into four types: (1) Marginal fibers, found throughout the peripheral pulp, constitute most of the nerve endings and usually do not reach the predentin. (2) Simple predentinal fibers which reach as far as the predentin. (3) Complex predentinal fibers which arborize profusely within the predentin. (4) Dentinal fibers, the rarest of all, are nerves that pass through the predentin and enter the dentinal tubules, without any branching.
It is estimated that the percentage of dentinal tubules which contain nerve fibrils is 25 % in the pulp horns, 15 % in the coronal dentin, and 10 % in the radicular dentin (Holland 1981). Also, the nerve fibrils may extend up to 1 mm into the dentinal tubule (Lilja 1980). The nerve bundles that enter the tooth are known to consist of sensory afferent nerves, which arise from the trigeminal (5th cranial) nerve, as well as sympathetic branches that arise from the superior cervical ganglion. Some of these nerve fibers are myelinated and others are nonmyelinated. The sympathetic nerve fibers are known to modulate the microcirculation of the dental pulp by controlling the contractions of the smooth muscle cells of the arterioles and the precapillaries.
The sensory nerves in contrast are of two types: A (myelinated) and C (nonmyelinated) nerve fibers. The majority of A fibers are of the A-delta type, which has a diameter range from 1 to 4 μm; they conduct impulses in 4–30 m/s. About 1 % of the A fibers are of the A-beta type, which has a diameter of 6–12 μm and conducts impulses in a faster rate as high as 48 m/s (Narhi et al. 1982a). The unmyelinated C-type nerve fibers have a diameter range of 0.4–1.2 μm and conduct impulses at a rate of 0.5–2 m/s.
4.2.1 Clinical Implications for Intrapulpal Sensory Nerve Fibers
The A-delta fibers have a small diameter and therefore a slower conduction velocity than other types of A fibers, but their conduction velocity is faster than C fibers. The A fibers transmit pain directly to the thalamus, generating a fast, sharp pain that is easily localized. The C fibers are influenced by many modulating interneurons before reaching the thalamus, resulting in a slow pain, which is characterized as dull and aching. The A fibers respond to various stimuli such as probing, drilling, and hypertonic solutions through the hydrodynamic effect (Braennstroem and Astroem 1964; Andrew and Matthews 2000; Narhi 1985; Narhi et al. 1992a).
This effect depends on the movement of the dentinal fluid in the dentinal tubules in response to a stimulus. Although the normally slow capillary outward movement does not stimulate the nerve endings and cause pain (Matthews and Vongsavan 1994; Pashley 1990; Vongsavan and Matthews 2007), rapid fluid flow, as in the case of desiccating or drying dentin, is more intense and is likely to activate the pulpal nociceptors (Braennstroem and Astroem 1964).
Thermal stimuli cause fluid movement through the dentinal tubules, resulting in a painful sensation in a tooth with a viable sensory pulp (Trowbridge 1985, 2003). This response is due to the rapid temperature change that causes a sudden fluid flow within the tubules and deforms the cell membranes of the free nerve endings exciting the A-delta fibers. A gradual change in temperature, however, does not cause an immediate pain response because this elicits a response from the C fibers (Bender 2000; Trowbridge et al. 1980; Narhi et al. 1982b).
Application of cold decreases the blood flow because of its vasoconstrictive effect on the blood vessels. If this application is continued, anoxia results and the A fibers cease to function. With continuous application of heat, the C fibers are affected: vasodilation temporarily increases intrapulpal pressure and causes intense pain (Bender 2000).
Hypertonic solutions activate the intradental nerves through osmotic pressure (Narhi et al. 1992a; Vongsavan and Matthews 2007; Pashley 1986; Anderson et al. 1967), manifested clinically by the pain that results when saturated sucrose solutions come into constant contact with sensitive dentin. This sensitivity is a direct response to the stimulation of the A fibers. Another example is the use of an etchant on the dentinal surface. The osmotic pressure of the acid used for etching the dentin is as important as the acid’s chemical composition in the induction of pain because this osmotic pressure causes the outward fluid flow in the tubules, together with aspiration of the odontoblastic nucleus (Anderson et al. 1967; Narhi et al. 1992b; Narhi and Hirvonen 1987).
The ionic concentration of the material also affects the reduction of pain in the sensitive dentin. A normally irritant substance such as potassium chloride temporarily relieves pain because the high concentration of potassium temporarily blocks the conduction of nerve impulses, causing a hyperpolarization that decreases the excitability of the nerve fibers. This hyperpolarization is the basis for the addition of potassium ions to dentifrices to control dentin hypersensitivity.
4.3 Characteristics of Pain of Dentin Hypersensitivity and Associated Factors
Dentin hypersensitivity can be viewed as a symptom complex rather than a true disease. Clinically, it is not associated with obvious tissue damage, but the symptoms indicate potential damage, with constant erosion and attrition of enamel or cementum and a concomitant pulpal response (Curro 1990). The chief symptom of dentin hypersensitivity is pain characterized by rapid onset, sharpness, and short duration. Occasionally, it may persist for a variable time as a dull or vague sensation in the affected tooth after removal of the stimulus. Tactile (toothbrushing and digital probing), thermal (hot and cold), and chemical (acids and sweets) stimuli, as well as exposure to air, can elicit painful responses from individuals with hypersensitive teeth. Brannstrom and Astrom (1972) believed that pain from heat takes longer to develop than pain from cold because heat causes inward movement of the tubular fluid, while the outward movement caused by cold is more rapidly developing (Drisko 2002). Although at times pulpal inflammation complicates the symptomatology, dentin hypersensitivity differs from pain arising in the pulp due to inflammation. Patients can readily locate the source of discomfort or pain when a stimulus is applied to a hypersensitive tooth.
A patient that suffers from dentin hypersensitivity may perceive an exaggerated, intense pain or a more continuous pain stimulus of longer duration which would not be expected to originate only from a hydrodynamic stimulus. The latter is likely to be associated with pulp inflammation. This is because A fibers are relatively insensitive to inflammatory mediators, whereas C fibers may take active part in the development of pulpal inflammation (Olgart and Kerezoudis 1994). Stimuli such as heat, cold, osmotic change, and acid can start an episode of pulpal pain that may last several minutes to many hours and it is usually difficult to locate. Chewing can also be a source of stimulus for pulpal pain, due to hydraulic action, osmotic effects, or trauma.
Dentin hypersensitivity is usually a chronic condition with acute episodes; the dentin that is freshly cut or has recently been curetted will respond to the same cold, heat, osmotic, and acid stimuli in an acute manner. Following cavity preparation, or curettage, the dentin may “heal” due to mineral formation which blocks the tubules, but if “healing” does not occur, the tooth may respond chronically to stimuli that are usually not considered as noxious (Curro 1990).
The history of the patient’s pain is the first clinical data that the dentist must collect and consider. The dentist should pay careful attention to the patient’s answers about the pain, such as the type, duration, frequency, aggravating factors, effect of analgesics, and tenderness when biting. The nature of the pain described by most patients seeking treatment for dentin hypersensitivity is a sharp pain of short duration (Orchardson and Collins 1987a; Andrej 2002; Canadian Advisory Board on Dentin Hypersensitivity 2003).
The frequency of each episode of pain of dentin hypersensitivity is variable and dependent, somewhat, on the initiating stimulus and is patient dependent. Cold or heat has been described as the most potent stimuli (Canadian Advisory Board on Dentin Hypersensitivity 2003; Orchardson and Collins 1987b; Irwin and McCusker 1997; Rees 2000). The episodic pain associated with dentin hypersensitivity may last from months to years as reported by Schuurs et al. (1995). Taani and Awartani (2002) in a hospital-based study population of 302 subjects reported that 14–23 % claimed their sensitivity had lasted from 1 to 5 years.
Drinking cold water was one function that was severely interfered with in a study by Bamise et al. (2007) with slightly lower percentages of the volunteers mentioning brushing and eating. Taani and Awartani (2002) reported that about 64 % of their patients with dentin hypersensitivity reported that it did not interfere with eating and toothbrushing. The hypersensitivity reported when drinking cold water was explained by the fact that drinking water gains access to more sites in the mouth.
4.3.1 Gender Considerations in the Diagnosis of Dentin Hypersensitivity
Please refer to Sect. 1.3.
4.3.2 Age Range of Sufferers of Dentin Hypersensitivity
Please refer to Sect. 1.3.
4.3.3 Side and Site Predilections of Dentin Hypersensitivity
Please refer to Sect. 1.3.
4.3.4 Tooth Predilection for Dentin Hypersensitivity
Please refer to Sect. 1.3.
4.3.5 Relationship of Dentin Hypersensitivity and Plaque Scores
Please refer to Sect. Lack of Toothbrushing in Chap. 3.
4.4 Diagnostic Considerations for Causative Factors of Dentin Hypersensitivity
Management of a patient suffering from dentin hypersensitivity should be based on a correct diagnosis of the condition by the dentist, who should be aware of other clinical conditions (Sect. 4.3) which are similar in their presenting features (Dababneh et al. 1999). Conditions that lead to enamel loss and exposure of dentin should be carefully evaluated and diagnosed. As discussed earlier, in Sect. 3.3.1, these conditions place the teeth at a higher risk for DH.
Tooth surface loss caused by abfraction, abrasion, or erosion is assumed to progress slowly, a cumulative lifetime process, which is extremely difficult to diagnose in early stages. Sometimes, no obvious changes can be observed on the teeth for many years.
4.5 Differential Diagnosis of Dentin Hypersensitivity
The pain associated with hypersensitive teeth arises when the dentin is exposed and typically occurs in response to chemical, thermal, tactile, or osmotic stimuli. In patients with suspected dentin hypersensitivity due to positive findings in the screening and history, thorough differential diagnosis is very important to eliminate all other forms of orofacial pain.
Several conditions may elicit the same clinical symptoms as dentin hypersensitivity. These include:
-
Cracked tooth syndrome
-
Fractured restorations
-
Chipped teeth
-
Caries
-
Post-restorative sensitivity
-
Palatal-gingival groove
-
Hypoplastic enamel
-
Improperly insulated metallic restorations
-
Teeth in acute hyperfunction
A careful differential diagnosis, therefore, is required to rule out alternative causes of pain. This should include, as mentioned earlier, a history and a thorough clinical and radiographic examination of the tooth in question, as well as, all adjacent teeth.
4.6 Induction of Pain of Dentin Hypersensitivity
Various methods and devices (Fig. 4.2) have been used to stimulate dentin hypersensitivity in the screening and assessment of severity in sufferers, but a suggestion has been made that at least two different stimuli should be used (Gernhardt 2013).
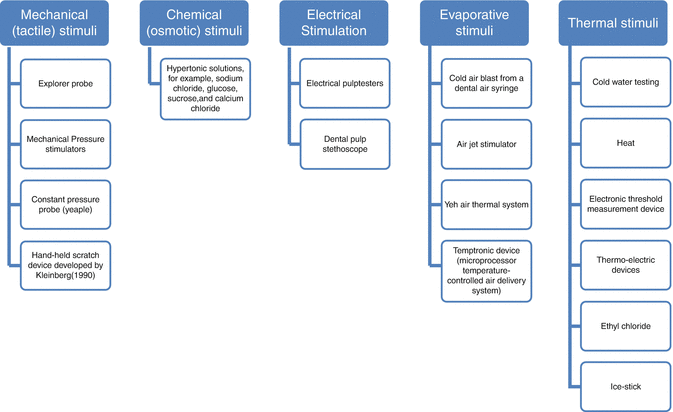

Fig. 4.2
Stimuli to assess dentin hypersensitivity (As classified by Gillam et al. 2000)
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


