Bisphosphonates are a class of agents used to treat osteoporosis and the complications associated with malignant bone metastases. Despite these benefits, osteonecrosis of the jaws has recently emerged as a significant complication in a subset of patients receiving these drugs. Based on a growing number of case reports and institutional reviews, bisphosphonate therapy may cause exposed and necrotic bone that is isolated to the jaw. This complication usually occurs following simple dentoalveolar surgery. The pathogenesis for this complication appears to be related to the profound inhibition of osteoclast function and bone remodeling. In this chapter, we review the clinical indications for bisphosphonate therapy along with the risks associated with their long-term use. Diagnostic strategies, risk stratification, and guidelines for staging and management of patients with established bisphosphonate-related osteonecrosis of the jaw (BRONJ) will also be reviewed.
BISPHOSPHONATES: WHAT ARE THEY AND HOW DO THEY WORK?
Bisphosphonates were developed in the nineteenth century, but were first investigated in the 1960s for use in disorders of bone metabolism. Their nonmedical use included water softening in irrigation systems used in orange groves. The initial rationale for their use in clinical practice was centered on their potential in preventing the dissolution of hydroxyapatite, the principal bone mineral, and hence arresting bone loss.
All bisphosphonates are nonmetabolized synthetic analogues of pyrophosphate. There are a variety of bisphosphonates that are approved for clinical use in the United States. They are segregated based on potency and route of administration ( Table 27-1 ).
| Generic Name | Brand Name (Delivery) | RAP * |
|---|---|---|
| Zoledronic acid | Zometa (IV) | 100,000 |
| Pamidronate | Aredia (IV) | 100 |
| Etidronate | Didronel (PO) | 1 |
| Tiludronate | Skelid (PO) | 10 |
| Alendronate sodium | Fosamax (PO) | 1000 |
| Risedronate | Actonel (PO) | 5000 |
| Ibandronate | Boniva (PO/IV) | 10,000 |
Bisphosphonates bind avidly to exposed bone mineral around resorbing osteoclasts, resulting in very high levels of bisphosphonates in the resorption lacunae. Since bisphosphonates are not metabolized, these high concentrations are maintained within bone for long periods of time. Bisphosphonates are then internalized by the osteoclast, causing disruption of osteoclast-mediated bone resorption at various levels. At the tissue level, bisphosphonates will inhibit bone resorption and decrease bone turnover as assessed by biochemical markers. The degree to which these compounds will also alter bone formation is related to their effects on bone turnover, which is closely coupled to bone formation. On a cellular level, the bisphosphonates are clearly targeting the osteoclasts and may inhibit their function in several ways: (1) inhibition of osteoclast recruitment, (2) diminishing the osteoclast life span, and (3) inhibition of osteoclastic activity at the bone surface. At a molecular level, it has been postulated that bisphosphonates modulate osteoclast function by interacting with a cell surface receptor or an intracellular enzyme.
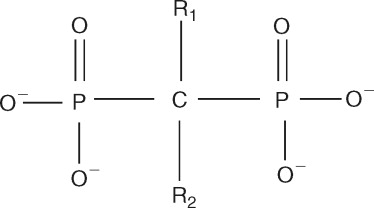
Each bisphosphonate has a different R1 or R2 side chain attached to the central carbon atom ( Figure 27-1 ). The R1 side chains determine their affinity for bone, and the R2 side chains are responsible for their antiresorptive properties. In general, side chains that contain a nitrogen atom are more potent than the nonnitrogen bisphosphonates. Etidronate, tiludronate, and clodronate are older generation bisphosphonates that do not contain nitrogen. Once internalized by the osteoclast, these agents inhibit adenosine triphosphate–dependent intracellular enzymes, which results in cellular apoptosis. The next generation of bisphosphonates contained a nitrogen side group characterized by a primary amine (alendronate and pamidronate) or a tertiary amine (risedronate, ibandronate). Zoledronic acid, the most potent bisphosphonate, is a tertiary amine that is part of a ring structure. These bisphosphonates inhibit the activity of the enzyme farnesyl diphosphate synthase in the mevalonate pathway, which blocks the phenylation of small signaling proteins (GTPase) that are required for osteoclast function and survival.
Bisphosphonates also have demonstrated effects unrelated to osteoclast inhibition. Pamidronate has been associated with an acute phase reaction characterized by fever and transient changes in various cytokine levels, such as interleukin-6, tumor necrosis factor-α, C-reactive protein, and elastase. More importantly, pamidronate was reported to significantly depress bone blood flow in rats. The mechanism of this effect may be attributable to a complex interaction of pamidronate with growth hormone and insulinlike growth factor 1, both of which are thought to play a role in the regulation of blood circulation in bones. Bisphosphonates can also inhibit endothelial cell function in vitro and in vivo. Those cells treated with bisphosphonates demonstrated decreased proliferation, an increased rate of apoptosis, and a decrease in capillary-tube formation. In that same study, there was a marked reduction in the number of blood vessels in pagetic bone marrow after bisphosphonate treatment when compared with pretreatment biopsies. Bisphosphonates have also demonstrated potent antiangiogenic properties as a result of their ability to significantly decrease circulating levels of vascular endothelial growth factor in breast cancer patients with bone metastases. Wood et al demonstrated the antiangiogenic properties of bisphosphonates on several levels: (1) potent inhibitor of vessel sprouting in a chick embryo model and (2) potent inhibition of angiogenesis induced by subcutaneous implants impregnated with bFGF in a murine model. These previously unrecognized antiangiogenic properties have generated interest in using bisphosphonates as potential antitumor agents.
BISPHOSPHONATE USE IN CLINICAL PRACTICE
The complications associated with metastatic bone disease, osteoporosis, and Paget’s disease are related to perturbations in osteoclast function. Therefore it is not surprising that bisphosphonates, which are potent inhibitors of osteoclast function, have demonstrated clinical efficacy in treatment of these diseases.
MALIGNANT BONE DISEASE
Their role in decreasing osteoclast-mediated lysis of bone in disease secondary to multiple myeloma, breast cancer, and other solid tumors has been well established in clinical trials. The efficacy of intravenous bisphosphonates in reducing bone pain, hypercalcemia, and skeletal complications has been extensively documented in patients with advanced breast cancer and multiple myeloma. Myeloma-related lytic disease is now understood to be secondary to increased osteoclastic activity and impaired osteoblastic activity. Myeloma cells are known to secrete both stimulators of osteoclast activation, such as receptor activator of nuclear-kB ligand (RANKL), and soluble molecules, such as dickkopf 1 (DKK), that inhibit osteoblastic activity. Bisphosphonates inhibit osteoclast function and therefore block the formation of “punched-out” lytic bony lesions and consequent manifestations of lytic bony disease. In patients with osteolytic metastases, oral bisphosphonate therapy is not as efficacious as the intravenous form in preventing skeletal complications and therefore is not indicated for this patient group.
Thus bisphosphonates are frequently administered to patients with osteolytic metastases, especially if there is a risk of significant morbidity. Based on clinical practice guidelines established by the American Society of Clinical Oncology, the use of bisphosphonates is considered the standard of care for treatment of: (1) moderate to severe hypercalcemia associated with malignancy and (2) metastatic osteolytic lesions associated with breast cancer and multiple myeloma in conjunction with antineoplastic chemotherapeutic agents.
Annually, about 1 million women worldwide will develop breast cancer. Of those progressing to advanced disease, close to 70% will develop bone metastases. This has resulted in a rampant use of these bisphosphonates in most medical oncology practices within the past several years.
OSTEOPOROSIS
Osteoporosis, a disease that is characterized by increased skeletal fragility and low bone mineral density, is responsible for more than 1.5 million fractures in postmenopausal women each year at an annual cost of $18 billion. It is estimated that 35% of postmenopausal Caucasian American women have some degree of osteoporosis in the hip, spine, or forearm and that 40% of these women will experience some type of osteoporotic fracture. This is in contrast to males over the age of 50 where only 13% are estimated to have an osteoporotic fracture. Whereas bone quality cannot be measured directly, bone mineral density is a relatively easy parameter to measure. Osteoporosis is characterized clinically by the T score, which represents the number of standard deviations the bone mineral density is above or below the normal mean bone density of a young Caucasian individual.
As a potent suppressor of osteoclast activity, bisphosphonates slow the remodeling process and increase bone mineral density, thereby reducing the risk of fracture in women with osteopenia and osteoporosis. All bisphosphonates currently approved for osteoporosis treatment have been shown to significantly reduce the risk of osteoporotic fractures. Alendronate has been shown to prevent bone loss at the spine and hip in menopausal women and reduce fractures at these sites by approximately 50%. In a large prospective trial, risedronate produced a 30% reduction in hip fractures. As a result of their proven clinical efficacy, bisphosphonates are considered first-line therapy in the treatment of osteoporosis and are the most widely prescribed antiresorptive agent.
BISPHOSPHONATE USE IN CLINICAL PRACTICE
The complications associated with metastatic bone disease, osteoporosis, and Paget’s disease are related to perturbations in osteoclast function. Therefore it is not surprising that bisphosphonates, which are potent inhibitors of osteoclast function, have demonstrated clinical efficacy in treatment of these diseases.
MALIGNANT BONE DISEASE
Their role in decreasing osteoclast-mediated lysis of bone in disease secondary to multiple myeloma, breast cancer, and other solid tumors has been well established in clinical trials. The efficacy of intravenous bisphosphonates in reducing bone pain, hypercalcemia, and skeletal complications has been extensively documented in patients with advanced breast cancer and multiple myeloma. Myeloma-related lytic disease is now understood to be secondary to increased osteoclastic activity and impaired osteoblastic activity. Myeloma cells are known to secrete both stimulators of osteoclast activation, such as receptor activator of nuclear-kB ligand (RANKL), and soluble molecules, such as dickkopf 1 (DKK), that inhibit osteoblastic activity. Bisphosphonates inhibit osteoclast function and therefore block the formation of “punched-out” lytic bony lesions and consequent manifestations of lytic bony disease. In patients with osteolytic metastases, oral bisphosphonate therapy is not as efficacious as the intravenous form in preventing skeletal complications and therefore is not indicated for this patient group.
Thus bisphosphonates are frequently administered to patients with osteolytic metastases, especially if there is a risk of significant morbidity. Based on clinical practice guidelines established by the American Society of Clinical Oncology, the use of bisphosphonates is considered the standard of care for treatment of: (1) moderate to severe hypercalcemia associated with malignancy and (2) metastatic osteolytic lesions associated with breast cancer and multiple myeloma in conjunction with antineoplastic chemotherapeutic agents.
Annually, about 1 million women worldwide will develop breast cancer. Of those progressing to advanced disease, close to 70% will develop bone metastases. This has resulted in a rampant use of these bisphosphonates in most medical oncology practices within the past several years.
OSTEOPOROSIS
Osteoporosis, a disease that is characterized by increased skeletal fragility and low bone mineral density, is responsible for more than 1.5 million fractures in postmenopausal women each year at an annual cost of $18 billion. It is estimated that 35% of postmenopausal Caucasian American women have some degree of osteoporosis in the hip, spine, or forearm and that 40% of these women will experience some type of osteoporotic fracture. This is in contrast to males over the age of 50 where only 13% are estimated to have an osteoporotic fracture. Whereas bone quality cannot be measured directly, bone mineral density is a relatively easy parameter to measure. Osteoporosis is characterized clinically by the T score, which represents the number of standard deviations the bone mineral density is above or below the normal mean bone density of a young Caucasian individual.
As a potent suppressor of osteoclast activity, bisphosphonates slow the remodeling process and increase bone mineral density, thereby reducing the risk of fracture in women with osteopenia and osteoporosis. All bisphosphonates currently approved for osteoporosis treatment have been shown to significantly reduce the risk of osteoporotic fractures. Alendronate has been shown to prevent bone loss at the spine and hip in menopausal women and reduce fractures at these sites by approximately 50%. In a large prospective trial, risedronate produced a 30% reduction in hip fractures. As a result of their proven clinical efficacy, bisphosphonates are considered first-line therapy in the treatment of osteoporosis and are the most widely prescribed antiresorptive agent.
BISPHOSPHONATE-RELATED OSTEONECROSIS OF THE JAWS (BRONJ)
PATHOPHYSIOLOGY AND RISK FACTORS
Since 2003 numerous reports have been published highlighting the adverse effect profile of this class of agents including the development of osteonecrosis of the jaws in patients treated with bisphosphonates. Although the exact mechanism of bisphosphonate-induced osteonecrosis has not yet been determined, several hypotheses have been proposed. In most cases, the pathogenesis of this process is consistent with a defect in jawbone physiologic remodeling or wound healing. The profound inhibition of osteoclast function can also inhibit normal bone turnover to an extent that local microdamage from normal mechanical loading or injury (tooth extraction) cannot be repaired. Alternatively the antiangiogenic properties of bisphosphonates may affect the local bone blood supply, contributing to the apparent ischemic changes noted in the affected patients’ jawbones. Since only a minority of bisphosphonate users develop bone necrosis, it is also possible that individual genetic variations in drug metabolism or skeletal homeostasis may confer susceptibility or resistance to developing BRONJ.
The apparent selective involvement of the maxilla and mandible may be a reflection of the unique environment of the oral cavity. Typically, healing of an open bony wound (e.g., extraction socket) in the presence of normal oral microflora occurs quickly and without complication. However, when the healing potential or the vascular supply of the mandible or maxilla is compromised, either by tumoricidal radiation doses or some other agent(s) or pathologic process, minor injury or disease in these sites increases the risk of osteonecrosis and possible secondary osteomyelitis. Also bisphosphonates are preferentially deposited in bones with high turnover rates; given that the maxilla and mandible are sites of significant remodeling, it is possible that the levels of bisphosphonates within the jaw are selectively elevated.
Several retrospective clinical studies have identified potential risk factors associated with the development of BRONJ. These include a history of dentoalveolar trauma, duration of bisphosphonate exposure, and the type of bisphosphonate. In the majority of BRONJ cases reported to date, recent dentoalveolar trauma was the most prevalent and consistent risk factor. Patients with a history of inflammatory dental disease (e.g., periodontal and dental abscesses) are at a sevenfold increased risk of developing BRONJ. This underscores the importance of maintaining good oral health and avoiding extractions in this population. The duration of bisphosphonate therapy also appears to be related to the likelihood of developing necrosis, with longer treatment regimens associated with a greater risk of developing disease. In addition, the more potent intravenous bisphosphonates, such as pamidronate and especially zoledronate, appear to be significantly more problematic as compared with the oral bisphosphonate medications. Initially, BRONJ was seen only with the use of the more potent intravenous forms of the drug; however, there have been reports of osteonecrosis in patients on the less potent oral forms. This alarming finding may have significant implications as the number of patients on oral bisphosphonates increases. Though found in both sexes, the literature reports more cases of BRONJ in females than males, which is likely a reflection of the large number of cases reported in breast cancer patients. With postmenopausal osteoporosis as an indication for bisphosphonate use, a large percentage of the female population may also be at risk of developing BRONJ. Patients receiving oral bisphosphonate therapy for osteoporosis who develop BRONJ have typically been exposed to these agents for a longer period of time (greater than 3 years) or were also exposed to steroid therapy.
Current incidence data for BRONJ are limited to retrospective studies with limited sample sizes. The current difficulty in establishing exact incidence data is due to several factors, which include a nonstandardized definition and inconsistencies in case recognition and reporting. With that understanding, the estimate of the cumulative incidence of BRONJ in patients receiving intravenous bisphosphonates for malignant disease ranges from 0.8% to 12%. For those patients exposed to oral bisphosphonates, the incidence appears to be significantly less.
In 2005 the Food and Drug Administration responded to the growing number of BRONJ cases by issuing a broad drug class warning of this complication for all bisphosphonates.
This has also prompted a change in clinical practice. With the benefit of bisphosphonate therapy beyond 5 years coming into question for patients with osteoporosis and the growing concern about long-term suppression of bone turnover, some clinicians have emphasized the importance of a drug holiday. In a consensus statement from the Mayo Clinic, the use of bisphosphonates in the treatment of multiple myeloma was modified to limit the exposure of intravenous bisphosphonates and minimize the potential for BRONJ.
CLINICAL PRESENTATION
The patient history and clinical examination are the most sensitive diagnostic tools for this condition. To distinguish BRONJ from other delayed intraoral healing conditions, the following definition has been proposed : A patient may be considered to have BRONJ if all of the following clinical conditions are present: (1) current or previous treatment with a bisphosphonate; (2) exposed, necrotic bone in the maxillofacial region that has persisted for more than 8 weeks; and (3) no history of radiation therapy to the jaws .
Areas of exposed and necrotic bone may remain asymptomatic for weeks, months, or years and may result in pain or exposed maxillary or mandibular bone. These lesions are most frequently symptomatic when surrounding tissues become inflamed or there is clinical evidence of exposed bone. Signs and symptoms that may occur before the development of clinically detectable osteonecrosis include pain, tooth mobility, mucosal swelling, erythema, and ulceration. These may occur spontaneously or more commonly at the site of prior dentoalveolar surgery. Intraoral and extraoral fistulae may develop when necrotic jawbone becomes secondarily infected ( Figure 27-2 ). Some patients may also have complaints of altered sensation in the affected area as the neurovascular bundle becomes compressed from the inflamed surrounding bone. Chronic maxillary sinusitis secondary to osteonecrosis with or without an oral-antral fistula can be the presenting symptom in patients with maxillary involvement. It has been observed that lesions are found more commonly in the mandible than the maxilla (2 : 1 ratio) and more commonly in areas with thin mucosa overlying bony prominences, such as tori, bony exostoses, and the mylohyoid ridge. The size of the affected area can range from a nonhealing extraction site to necrosis of the entire jaw.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


