4 Development of the dentition
Prenatal development of the dentition
The anatomy of tooth development
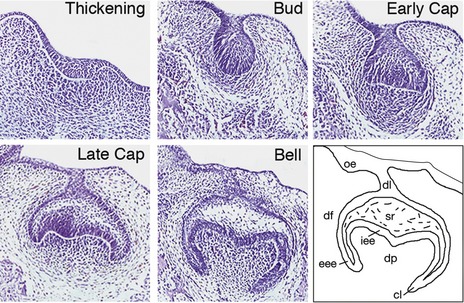
Discreet swellings of the dental lamina form the enamel organs of the future developing teeth. Epithelial cells of the enamel organ proliferate and progress through characteristic bud, cap and bell stages. Simultaneously, the dental papilla is formed by localized condensation of neural crest-derived ectomesenchymal cells around the epithelial invaginations. More peripherally, ectomesenchymal cells extend around the enamel organ to form the dental follicle. Together, these tissues constitute the tooth germ and will give rise to all structures that make up the mature tooth (Fig. 4.1).
The permanent dentition replaces the deciduous dentition and is composed of both successional and accessional teeth (Fig. 4.2):
The molecular control of tooth development
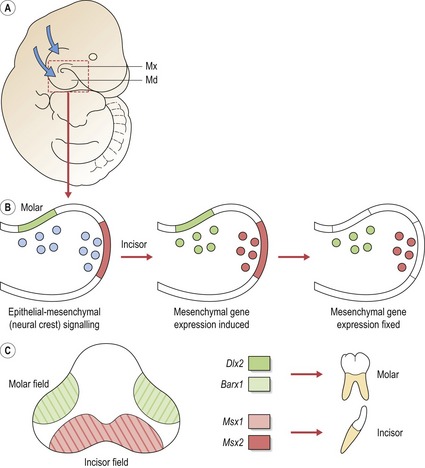
The histological basis of tooth development has been understood for some time, but in recent years progress has been made in understanding molecular mechanisms that underlie the process of odontogenesis using mouse models (Box 4.1). The generation of a tooth requires coordinated molecular signalling between epithelium of the early jaws and the underlying neural crest cells that migrate into these regions (Cobourne & Sharpe, 2003; Tucker & Sharpe, 2004).
Patterning the dentition: a molecular code for tooth shape
Initially, signalling from the epithelium to neural crest cells that migrate into the jaws can induce expression of a range of genes. However, expression domains very rapidly become established and then independent of epithelial signalling. It has been suggested that the differing combinations of gene expression patterns act to specify tooth shape (Sharpe, 1995). The ‘odontogenic homeobox code’ predicts that for each tooth-forming region of the early maxilla and mandible, the morphology of the developing tooth is dictated by a specific combination of homeobox genes within the ectomesenchyme (Box 4.2). Thus, for the molar region of mouse jaws, an overlapping code of Barx1 and Dlx2 exists (Fig. 4.3). There are several important points to note with regard to the homeobox model (Sharpe, 2001):
Box 4.2 Transformation of tooth type by manipulation of homeobox genes
An incisor tooth germ has been converted into one demonstrating a molar crown shape by manipulating homeobox gene expression in the future incisor-forming region of the mouse jaw. Barx1 is normally expressed in molar-forming mesenchyme of the jaws, this expression being established by Fgf8 signalling from the overlying epithelium. Barx1 expression is restricted to the molar regions by antagonistic signalling from Bmp4, which is present in the incisor-forming epithelium and represses Barx1 in the underlying mesenchyme. By artificially inhibiting Bmp4 activity in the incisor region of cultured jaws, the expression of Barx1 can be extended into the incisor region. Moreover, Bmp4 normally induces Msx1 in the incisor mesenchyme; therefore a loss of Bmp4 in this region also reduces expression of Msx1. The code of these early incisor regions is therefore altered into one resembling a molar region: a gain of Barx1 and loss of Msx1. Transplantation of these early incisor regions into sites that allow tooth development to progress towards completion results in the formation of multicusped molar teeth rather than incisors. Thus, an experimental alteration of homeobox gene expression can re-specify the identity of developing teeth, which is powerful evidence in support of the ‘odontogenic homeobox code’ (Tucker et al, 1998).
Initiation of tooth development
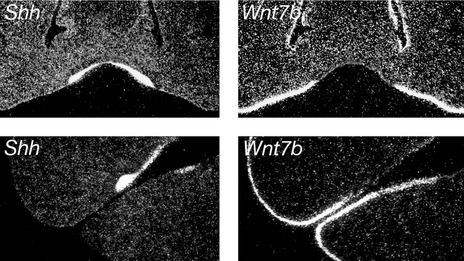
Once the ectomesenchyme within each mouse jaw has been regionalized into presumptive incisor and molar domains, tooth development is initiated within the jaw epithelium. A key player in this process is sonic hedgehog (Shh), a protein produced in localized regions of jaw epithelium where the teeth are going to form. Shh drives proliferation of the dental lamina within these regions, resulting in formation of the tooth buds; if Shh signalling is lost in the early dental lamina, teeth fail to develop. Restriction of Shh production is therefore important in ensuring that teeth develop in the correct regions of the jaws and this is orchestrated by molecular compartmentalization of the jaw epithelium into tooth-forming and non-tooth-forming regions. Specifically, expression of the Shh gene is restricted to the tooth-forming regions because it is repressed throughout the non-dental epithelium by another signalling molecule called Wnt7b. Therefore, tooth formation in the correct regions of the jaws is established via reciprocal expression domains between two different signalling molecules within the jaw epithelium (Fig. 4.4).
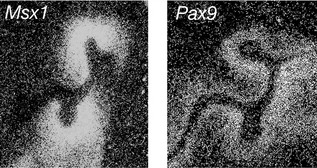
Once the tooth bud has formed, a number of homeobox-encoding genes subsequently localize to the condensing dental papilla, including Msx1 and Pax9. These genes play an important role in mediating later signalling between the underlying ectomesenchyme and the epithelial bud as tooth development progresses to the cap stage (Fig. 4.5). A loss of either gene leads to the arrest of tooth development at the bud stage in the mouse. Mutations associated with human MSX1 and PAX9 have also been implicated in hypodontia.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses