Dentoalveolar infections
Publisher Summary
Pus-producing or pyogenic infections which are associated with a tooth and surrounding supporting structures, such as the periodontal membrane, cementum and alveolar bone, have been described using a number of terms such as periapical abscess, apical abscess, chronic periapical dental infection, dental pyogenic infection, periapical periodontitis, and dentoalveolar abscess. In this chapter the last term will be used. The clinical presentation is variable and is related to the interaction of a number of factors such as the virulence of the causative microorganisms, the state of the local and systemic defence mechanisms of the host and a number of anatomical features. Depending on how these factors interact, the resulting infection may present as an abscess localized to the tooth which initiated the infection, as a diffuse cellulitis which spreads along fascial planes, or a mixture of both. The primary source of microorganisms in these infections is either from the apex of a necrotic tooth or from periodontal pockets. Usually the source of infection in the periapical region is from dental caries via the pulp chamber and root canal.
Dentoalveolar abscess
Aetiology
The usual stages in the development and spread of a dentoalveolar abscess are shown in Figure 6.1. Microorganisms present in the initial carious lesion extend into dentine and spread to the pulp via the dentinal tubules. The pulp responds to infection in a number of ways, varying from rapid acute inflammation involving the whole pulp which quickly becomes necrosed, to the development of a chronic localized abscess with most of the pulp remaining viable. It is not clear why these different responses occur but it is probably due to a combination of a number of factors, such as variation in the toxicity of different bacterial species, as well as in the specific and non-specific defence mechanisms of the host. Microorganisms may enter the pulp by routes other than through a carious lesion, e.g. invasion may occur as a result of tooth fracture or as a result of a traumatic exposure during dental treatment. Pulp death may occur as a result of trauma without fracture or by chemicals used incorrectly during restorative treatment. In these examples the dead pulp tissue is sterile but may subsequently become infected via the gingival lymphatics or as a sequel to a bacteraemia caused, for example, by tooth extraction at a different site.
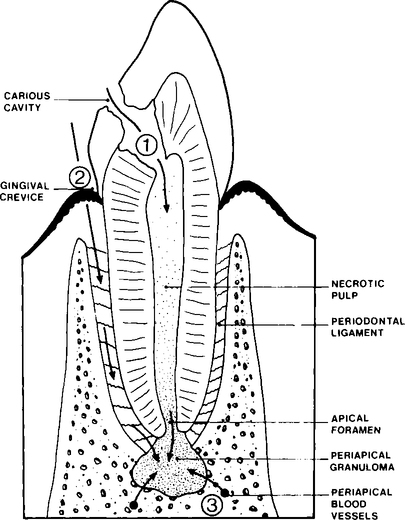
Infection and death of the pulp results in the pulp chamber and root canal becoming colonized by microorganisms. These microorganisms have a potential to produce a wide range of irritant substances, for example, enzymes, acids and toxins. Bacteria and their irritant products may leak from the root canal into the periapical tissues and stimulate an acute inflammatory response. As in the case of the dental pulp, the periapical tissues may react to infection in a number of ways depending on the virulence of the microorganisms involved and the efficiency of the hosts specific and non-specific defence mechanisms (see Chapter 3). However, the precise reasons why an acute abscess develops during periapical infection in some individuals but not others remain unclear.
Spread of infection and sequelae
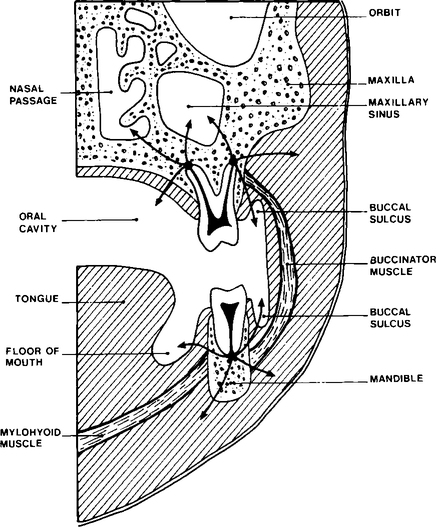
Pus, once formed, may remain localized at the root apex with the formation of a chronic abscess which can develop into a focal osteomyelitis, or may spread by various routes as shown in Figure 6.2. Pus may spread through the cortical bone of the jaws into the superficial soft tissues or, more rarely, into the adjacent fascial spaces. Once in the soft tissues the pus may (1) localize as a soft tissue abscess, (2) spread through the overlying oral mucosa or skin producing a sinus linking the main abscess cavity with the mouth or skin, or (3) extend through the soft tissue to produce a cellulitis. When dentoalveolar infection spreads deeply into soft tissues it tends to follow the path of least resistance, i.e. through connective tissue and along fascial planes. Extension of infection into deep fascial spaces is determined by the relation of the original abscess to the anatomy of the adjacent tissues. Students interested in the topic should consult a textbook on oral surgery. Infection via fascial planes often spreads rapidly and for some distance from the original abscess site and occasionally may cause severe respiratory distress, due to occlusion of the airway by oedema. Finally, pus may spread into the deeper medullary spaces of alveolar bone, producing a spreading osteomyelitis.
Clinical presentation
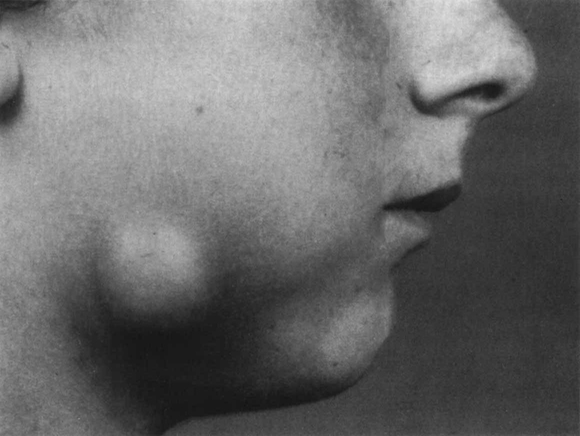
The presenting features of acute dental alveolar abscesses are very variable depending on (1) the site of infection, (2) the degree and mode of spread, (3) the virulence of the causative microorganisms, and (4) the efficiency of the host defence mechanisms. Usually a mixture of some or all of the following features are present: a non-viable tooth with or without a carious lesion; a large restoration; evidence of trauma; swelling; pain; redness; trismus; local lymph node enlargement; sinus formation; raised temperature and malaise due to toxaemia. A photograph of a typical dentoalveolar abscess is shown in Figure 6.3.
Microorganisms involved
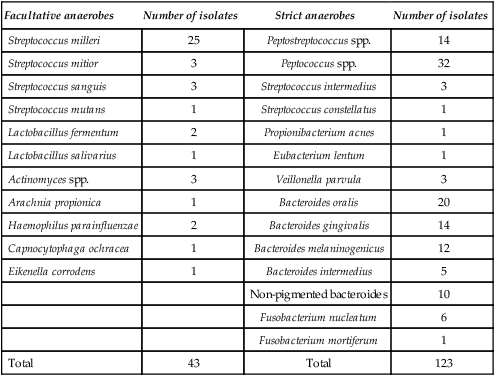
A microbiological investigation on a non-contaminated sample of pus from a dentoalveolar infection may yield a single isolate, a mixture of two to three different bacterial species, or a complex mixture of microorganisms involving perhaps eight different species. A single isolate is unusual and a mixture of 3–4 different species much more common. Many earlier reports dealing with the microbiology of dentoalveolar abscesses have undoubtedly underestimated the incidence of strictly anaerobic bacteria, probably due to a combination of inadequate sampling and transportion to the laboratory, together with poor laboratory technique. Well-controlled studies have shown that strict anaerobes are usually the predominant organisms and that ’Streptococcus viridans’ is less common than one would assume from many reports. While almost every member of the oral flora has been isolated from dentoalveolar infection at one time or another, there is little doubt that some bacterial species are more common than others. The common species isolated from non-specific abscesses are shown in Table 6.1 and it is clear that strict anaerobes predominate (especially Bacteroides spp. and anaerobic streptococci), although facultative bacteria, e.g. Strep. milleri, are also found both in pure and mixed culture.
Table 6.1
Identity of 166 bacterial strains isolated from 50 acute dentoalveolar abscesses
| Facultative anaerobes | Number of isolates | Strict anaerobes | Number of isolates |
| Streptococcus milleri | 25 | Peptostreptococcus spp. | 14 |
| Streptococcus mitior | 3 | Peptococcus spp. | 32 |
| Streptococcus sanguis | 3 | Streptococcus intermedius | 3 |
| Streptococcus mutans | 1 | Streptococcus constellatus | 1 |
| Lactobacillus fermentum | 2 | Propionibacterium acnes | 1 |
| Lactobacillus salivarius | 1 | Eubacterium lentum | 1 |
| Actinomyces spp. | 3 | Veillonella parvula | 3 |
| Arachnia propionica | 1 | Bacteroides oralis | 20 |
| Haemophilus parainfluenzae | 2 | Bacteroides gingivalis | 14 |
| Capnocytophaga ochracea | 1 | Bacteroides melaninogenicus | 12 |
| Eikenella corrodens | 1 | Bacteroides intermedius | 5 |
| Non-pigmented bacteroides | 10 | ||
| Fusobacterium nucleatum | 6 | ||
| Fusobacterium mortiferum | 1 | ||
| Total | 43 | Total | 123 |
Collection and transportation of samples
The ideal sample of pus for microbiological assessment is a non-contaminated fluid sample which is transported directly to the microbiology laboratory. Details of the methods of sampling dentoalveolar infections and transporting the samples to the laboratory are described in Chapter 13
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses