Microbiology of periodontal diseases
Publisher Summary
Periodontal diseases occur in all parts of the world and few individuals live out their natural life span without becoming affected. However, in the majority of individuals, the common chronic inflammatory diseases which involve the gingival and periodontal tissues can be controlled, if not cured, using mechanical cleansing techniques and encouraging good oral hygiene. A small but significant number of patients experience rapidly progressive disease which requires assessment and treatment by periodontologists.While there is no doubt that microorganisms play an important role in the aetiology of most forms of periodontal disease, there is dispute as to whether their involvement is of a specific or non-specific nature. At present, clinical and radiological examination are unable to diagnose active disease or to predict which patients are likely to experience severe progressive periodontitis. Tissue destruction can be recorded with certainty only in a retrospective manner. Therefore, much of the current research in periodontology is directed towards developing laboratory tests which will allow high-risk patients and active disease to be identified early and ensure that subsequent specialized treatment is effective. An area of current interest is AIDS-related periodontal disease which is described in Chapter 16.
Types of periodontal disease
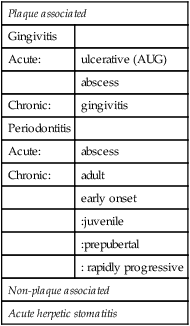
The main types of periodontal disease which are believed to have a microbial aetiology are shown in Table 5.1. However, as detailed knowledge about the aetiology of some of these conditions, e.g. chronic periodontitis, becomes available, it is likely that periodontal infections with apparently the same clinical signs and symptoms will turn out to be due to a number of different host–parasite interactions.
Table 5.1
Classirication of periodontal diseases with a microbial aetiology
| Plaque associated | |
| Gingivitis | |
| Acute: | ulcerative (AUG) |
| abscess | |
| Chronic: | gingivitis |
| Periodontitis | |
| Acute: | abscess |
| Chronic: | adult |
| early onset | |
| :juvenile | |
| :prepubertal | |
| : rapidly progressive | |
| Non-plaque associated | |
| Acute herpetic stomatitis | |
Factors involved in periodontal diseases
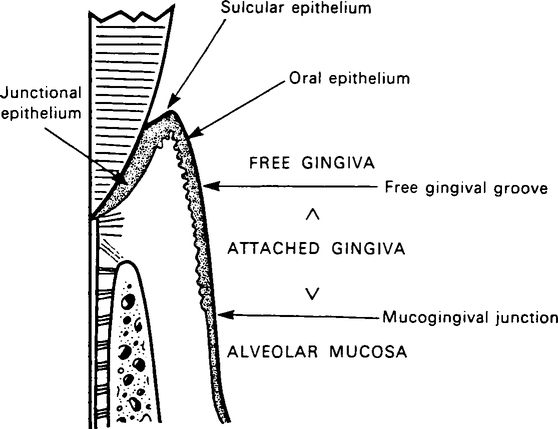
Host tissues
The term periodontium refers to the anatomical structures involved in resisting forces applied to the teeth, especially the gingivae, periodontal ligament, cementum and alveolar bone. The part of the periodontium which supports the coronal portion of the root is known as the marginal periodontium (Figure 5.1). A detailed description of these tissues and the changes which occur with the onset of disease is outside the scope of this book and students should consult standard periodontology textbooks. While the dentogingival junction is a site of potential weakness, the host defences are able to function effectively as long as oral hygiene is satisfactory (Table 5.2). However, when plaque is allowed to accumulate undisturbed and close to the gingival margin, the host defences become stressed.
Table 5.2
Specific and non-specific factors present in the gingival crevicular fluid
| Specific | Non-specific |
| B and T lymphocytes | Polymorphonuclear and mononuclear phagocytes |
| Antibodies: IgG, IgM and IgA | Complement components |
| Lysozyme | |
| Enzymes from host |
Host defence factors
One of the important components of the host response is the gingival crevicular fluid (GCF) which contains both specific and non-specific factors (Table 5.2). Although it is convenient to describe these factors separately, it is important to remember that in vivo they interact closely, e.g. antibodies can act as opsonins thereby potentiating phagocytic activity. The nature of the non-specific host factors are shown in Table 5.3.
Table 5.3
Non-specific defence mechanisms in the marginal periodontium
| Host cells | Gingival crevicular fluid |
| Anatomical epithelial seal in the base of the gingival sulcus | Mechanical washing action; tendency to cleanse crevice |
| Rapid repair of junctional epithelium following injury | Antimicrobial factors, e.g. lysozyme |
| Shedding of surface cells with attached bacteria from sulcular epithelium into crevice | |
| Phagocytosis together with migration of polymorphonuclear and mononuclear phagocytes through the junctional epithelium |
Antibody
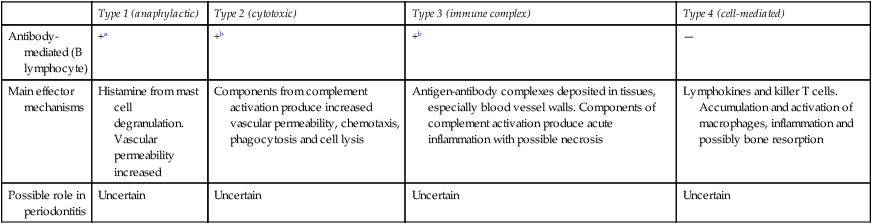
Since antibodies and complement are present in the periodontal tissues, hypersensitivity reactions which could result in damage to host tissues may contribute to periodontal disease. While there is evidence that all four types of hypersensitivity may be involved under certain circumstances, their precise role in pathogenesis or in recovery remains uncertain (Table 5.4).
Table 5.4
Possible hypersensitivity mechanisms in periodontitis
| Type 1 (anaphylactic) | Type 2 (cytotoxic) | Type 3 (immune complex) | Type 4 (cell-mediated) | |
| Antibody-mediated (B lymphocyte) | +a | +b | +b | — |
| Main effector mechanisms | Histamine from mast cell degranulation. Vascular permeability increased | Components from complement activation produce increased vascular permeability, chemotaxis, phagocytosis and cell lysis | Antigen-antibody complexes deposited in tissues, especially blood vessel walls. Components of complement activation produce acute inflammation with possible necrosis | Lymphokines and killer T cells. Accumulation and activation of macrophages, inflammation and possibly bone resorption |
| Possible role in periodontitis | Uncertain | Uncertain | Uncertain | Uncertain |
Microbial factors
1. Worldwide epidemiological studies have shown a strong positive association between plaque and the prevalence and severity of periodontal deseases.
2. Clinical studies in patients with a healthy periodontium have shown that, if oral hygiene is discontinued, the accumulation of dental plaque which occurs is paralleled by the onset of gingivitis. If plaque is then removed and normal oral hygiene reintroduced, the tissues are restored to health.
3. The topical application of certain antimicrobial agents both inhibit plaque formation and prevent the development of gingivitis.
4. Certain bacteria isolated from human dental plaque, e.g. F. nucleatum, B. gingivalis and Eik. corrodens, are periodontopathic in gnotobiotic animals.
The microbial composition of the gingival crevice area in health and disease is shown in Table 5.5. The flora associated with health consists mainly of streptococci and Actinomyces spp. This changes both quantitatively and qualitatively during the development of disease, and differences can be demonstrated in plaque samples from healthy and diseased sites, as well as between different types of periodontal diseases (for details, see below under specific diseases).
Table 5.5
Microorganisms associated with various forms of periodontal disease
| Health/Disease | Predominant microorganisms | Comments |
| Health |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses