Fig. 15.1
Schematic drawing of the hydrodynamic mechanisms causing dentin hypersensitivity. Excitation at the dentin surface (i.e., root surface) causes an inward or outward flow of the intratubular fluid exciting the pulp nerve fibers (Courtesy of Dr. Luc M Martens)
Regarding the hydrodynamic mechanism, dentin will only be sensitive if the tubules are patent from the pulp to the oral environment (Fig. 15.2). Sensitive teeth have up to eight times more and up to two times wider tubules at the buccal cervical area as compared to nonsensitive teeth [13]. It has also been shown that smear layers in sensitive dentin are thinner and less calcified as compared to those of nonsensitive dentin [14]. As the patency will change with production and removal of the smear layer, episodic conditions are possible [13]. Spontaneously occurring changes in the exposed dentin, which in many cases seem to block the tubules, may reduce the responses to hydrodynamic stimulation and, thus, have an alleviating effect on dentin sensitivity.
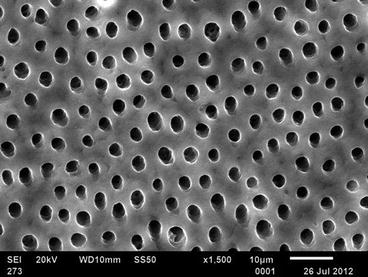

Fig. 15.2
Open tubules are a prerequisite for dentin hypersensitivity in most cases (Courtesy of Dr. Bennett T. Amaechi)
15.2.1 Predisposing Factors
15.2.1.1 Gingival Recession Exposing Dentin
To be hypersensitive, dentin must be exposed and the exposed tubules must be open and patent to both the oral cavity and the pulp [10, 15]. Exposure of dentin through the loss of gingival and periodontal tissue may be caused by either too meticulous or by neglected oral hygiene (Fig. 15.3). The exact mechanism by which too meticulous oral hygiene causes loss of tissue is not very well understood and often implies brushing force and brush bristle characteristics. Several studies have shown the injury potential of sharp nonrounded filament tips on gingival abrasion [16, 17]. Surprisingly there is no information on the role of toothpastes in this process. Such a role could be both physical, through abrasion, and chemical, through cytotoxicity of ingredients such as detergents to the soft tissues [18]. In any case, modifying factors like inserting frenula, thin gingival biotypes, a lack of keratinized gingiva, or absence of the buccal bone may be implicated and should be considered. The mechanism by which neglected oral hygiene causes recessions runs through acute and chronic periodontal diseases and nonsurgical and surgical treatments.
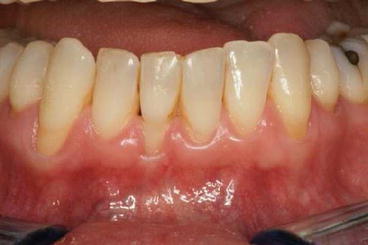

Fig. 15.3
Exposure of dentin through the loss of gingival and periodontal tissue may be caused by either too meticulous or neglected oral hygiene (Courtesy of Dr. Luc M Martens)
15.2.1.2 Loss of Hard Tissue Exposing Dentin
Exposure of dentin by the loss of enamel is often ascribed to abrasion. However, most abrasives are softer than enamel and it must be concluded that toothpaste abrasion alone would play a clinically insignificant role in exposure of dentin [18]. In contrast, acids from intrinsic or extrinsic sources are more harmful for enamel by dissolution and by softening. The softened enamel is subsequently abraded away by mechanical forces. Shear forces of the oral soft tissues may be sufficient to abrade the softened enamel [19], but toothbrushing surely will as will grinding and clenching [20]. So when there is exposure of dentin as a result of loss of enamel, the patient’s history should reveal the role of intrinsic or extrinsic acids (Table 15.1).
Table 15.1
Patient history
|
Ask patient to describe pain (look for description of pain as short, sharp)
|
|
Ask patient to identify pain-inciting stimuli (thermal, tactile, evaporative, osmotic, chemical)
|
|
Determine patient’s desire for treatment
|
|
Probe for lifestyle habits/practices, intrinsic and extrinsic acid (citrus juices and fruits, carbonated drinks, wines, ciders)
|
|
Obtain detailed dietary information including dietary intake relevant to medical problems
|
|
Probe for gastric acid reflux and excessive vomiting
|
Since nonsensitive dentin reveals few if any open dentinal tubules at the surface [13], it is assumed that the tubules are covered by a “smear layer,” consisting of protein components and calcium phosphate deposits derived from saliva [23] (Fig. 15.4). To initiate dentin hypersensitivity this layer has to be removed, and in vitro and in situ studies implicate erosive wear, as the smear layer is sensitive to acids [24, 25]. When acids have softened the smear layer and dentin, the materials are more susceptible to physical forces, such as toothbrushing. Clinical data suggest that physical forces alone are not a key factor in removing the smear layer and opening exposed dentin tubules [10]. Also toothpaste will remove the smear layer [25, 26] probably by a combined abrasive and detergent action. Moore and Addy [27] have suggested that certain “mild” surfactants and “gentle” abrasives might have advantages over their more traditional counterparts in toothpastes marketed for the relief of dentin hypersensitivity [27]. However, this hypothesis does not appear to have been clinically validated in well-designed clinical studies [15]. One study showed no difference in desensitizing effect after elicitation using the evaporative method when using four desensitizing toothpastes different in abrasivity with RDA 60, 108, 150, or 210, respectively [28].
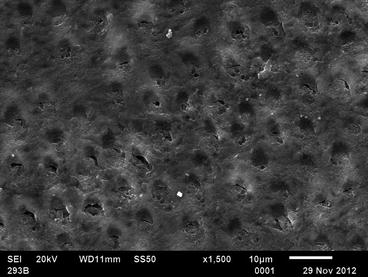

Fig. 15.4
Dentin surface of Fig. 15.2 covered by a smear layer (Courtesy of Dr. B Amaechi)
Subsequent to tubule exposure, toothpaste may reduce patency by secondary abrasive smearing or deposition of toothpaste constituents onto the dentin surface and into tubules. This makes the role of toothpaste without active ingredients to reduce dentin hypersensitivity inconclusive, even of fluoride containing pastes. Additionally it suggests that when using desensitizing toothpastes application with a fingertip or cotton swab after brushing may be beneficial.
15.3 Clinical Assessment
Clinical examination for dentin hypersensitivity would include a pain provocation test. However, the patient’s perception of dentin hypersensitivity is subjective and clinical evaluation based on any scoring or rating system regarding its severity is challenging. Nevertheless, it is important to detect, rate, and monitor the pain as accurately as possible in order to define the baseline status and to observe any changes in due course and after therapy. Ideally, the latter ends in a status where “no pain” can be attested, but this ideal dichotomous treatment goal is still difficult to achieve.
Provocation tests are most frequently used to simulate pain and to assess the immediate reaction:
1.
Tactile stimulus. This is the use of a probe, which is used as a “scratch” test on the exposed dentin, preferably with a standardized pressure. The use of probes would be contraindicated in evaluating treatments that use adhesive restorative materials, or other barrier methods. In such cases, the use of controlled air stimuli, graded cold water, or contact cold probes would be more appropriate [29].
2.
An evaporative air stimulus. The Schiff Cold Air Sensitivity Scale is frequently used to assess the subject response to the air blast hypersensitivity [30]. This scale is scored as follows:
-
0 = Subject does not respond to air stimulus.
-
1 = Subject responds to air stimulus but does not request discontinuation of stimulus.
-
2 = Subject responds to air stimulus and requests discontinuation or moves from stimulus.
-
3 = Subject responds to air stimulus, considers stimulus to be extremely painful, and requests discontinuation of the stimulus.
Noteworthy, the teeth on either side of the tooth under investigation should be isolated so that no referred pain is detected.
3.
A cold stimulus, which can be graded cold water or contact cold probes.
After this pain induction, either a scoring system such as “Dental Pain Scale (DPS)” rates the pain answer or a visual analogue scale (VAS) can be used to “quantify” the severity in millimeter (mm) (Fig. 15.5).
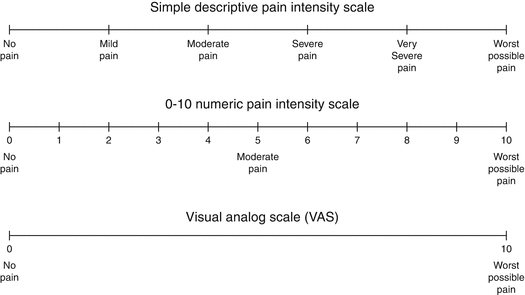

Fig. 15.5
Frequently used scales for pain intensity measurement
Often individuals will not respond to all types of stimulus or may respond differently to different stimuli [31–33], so it is recommended that at least two hydrodynamic stimuli should be used. The interval between stimulus applications should be of sufficient duration to minimize interactions between stimuli. If multiple stimuli are used to help to achieve a diagnosis, the order of application should be that which causes the least to the most amount of pain [34]. Repeated testing should be avoided as it is not known how long it takes to reach threshold evaluation. In case of a negative provocation test, any dentin hypersensitivity therapy becomes needless.
It is also questionable to what extent therapies should be performed in cases where patients display with no self-reported pain but show the typical signs of dentin hypersensitivity during a routine clinical examination. In fact, prophylactic measures, which protect the exposed surfaces against cariologic and wear challenges, may be considered, but the patient’s awareness of nonexisting subjective pathologic conditions should not be stimulated.
15.3.1 Oral Health-Related Quality of Life
Dentin hypersensitivity may disturb the patient during eating, drinking, toothbrushing, and sometimes even breathing. The resulting restrictions on everyday activities can have an important effect on the patient’s quality of life [35]. Oral health-related quality of life (OHRQoL) is a relatively new concept in dentistry. It is an aspect of dental health addressing the patient’s perception of whether his/her current oral health status has an impact upon his/her actual quality of life [35]. Therefore, OHRQoL may provide a new perspective when looking at a patient, by measuring treatment efficacy in terms of patient satisfaction. There is only little research into the relevance of the various quality of life questionnaires in the treatment of dentin hypersensitivity, yet it may be very valuable to the patient to evaluate the treatment according to these values. Boiko et al. [36] developed, based on in-depth and focus group interviews, a dentin hypersensitivity experience questionnaire (Table 15.2) to capture subjective impacts on patients. The questions can be phrased like “Having the sensations in my teeth takes a lot of the pleasure out of eating and drinking” after which the patients can indicate to what extent he agrees or disagrees.
Table 15.2
The items of the dentin hypersensitivity experience questionnaire developed by Boiko et al. [36] to determine the impact of dentin hypersensitivity on a patient’s quality of life
|
1 disagree strongly
|
2 agree a little
|
3 agree
|
4 agree moderately
|
5 agree strongly
|
||
|---|---|---|---|---|---|---|
|
Restrictions
|
Pleasure out of eating
|
|||||
|
Cannot finish meal
|
||||||
|
Longer to finish meal
|
||||||
|
Problems with eating ice-cream
|
||||||
|
Adaptation
|
Modification of eating
|
|||||
|
Careful when breathing
|
||||||
|
Warming food/drinks
|
||||||
|
Cooling food/drink
|
||||||
|
Cutting fruit
|
||||||
|
Putting a scarf over mouth
|
||||||
|
Avoiding cold drinks/foods
|
||||||
|
Avoiding hot drinks/foods
|
||||||
|
Avoiding contact with certain teeth
|
||||||
|
Change toothbrushing habits
|
||||||
|
Biting in small pieces
|
||||||
|
Avoiding other food
|
||||||
|
Social
|
Longer than others to finish
|
|||||
|
Choose food with others
|
||||||
|
Hide the way of eating
|
||||||
|
Unable to take part in conversations
|
||||||
|
Painful at the dentist
|
||||||
|
Emotions
|
Frustrated not finding a cure
|
|||||
|
Anxious of eating contributes
|
||||||
|
Irritating sensations
|
||||||
|
Annoyed with myself for contributing
|
||||||
|
Guilty for contributing
|
||||||
|
Annoying sensations
|
||||||
|
Embarrassing sensations
|
||||||
|
Anxious because of sensations
|
||||||
|
Identity
|
Difficult to accept
|
|||||
|
Different from others
|
||||||
|
Makes me feel old
|
||||||
|
Makes me feel damaged
|
||||||
|
Makes me feel unhealthy
|
||||||
15.4 Differential Diagnosis
A number of other dental conditions can give rise to pain symptoms, which may mimic those of dentin hypersensitivity. Therefore, careful examination is necessary to exclude the following conditions, which need a variety of different treatment options [6, 22, 37, 38]:
-
Cracked tooth syndrome
-
Incorrect placement of dentin adhesives in restorative dentistry, leading to nanoleakage
-
Fractured restorations and incorrectly placed dentin pins
-
Inappropriate application of various medicaments during cavity floor preparation
-
Lack of care while contouring restorations so the tooth is left in traumatic occlusion
-
Pulpal response to caries and recent restorative treatment
-
Palatogingival groove and other enamel invaginations and defects
-
Chipped/fractured teeth causing exposed dentin
-
Tooth bleaching
-
Acute periodontal infections (e.g., necrotizing gingivitis/periodontitis or abscesses)
15.5 Preventive Strategies
Prevention is always better than cure. Thus, primary prevention represents the first line of defense against dentin exposure, i.e., the formation of gingival recession and dental hard tissue deterioration. Careful oral hygiene instructions and dietary advices are crucial. When dentin is already exposed, patients should be instructed in order to minimize the risk of opening the tubules and, thus, increasing the patency. Suggestions for patients and the dental professionals to avoid aggravating behavior or iatrogenic damage developed by Martens [22] are given in Table 15.3.
|
Suggestions for patients
|
|
Limit dietary acids
|
|
Use soft-medium toothbrush and adequate brushing technique
|
|
Use additional topical fluorides
|
|
Avoid picking, scratching at the gingival margins
|
|
Avoid excessive flossing or improper use of toothpicks
|
|
Suggestions for dental professionals
|
|
Avoid overinstrumentation of the root surfaces during scaling
|
|
Avoid excessive polishing of exposed dentin during stain removal
|
|
Avoid burning the gingival tissues during in-office bleaching
|
|
Advise patients to be careful during home-bleaching
|
|
Avoid harmful instruments and materials
|
15.6 Treatment Strategies
When the patients do suffer from dentin hypersensitivity, there is broad range of treatment options comprising home-use and professional approaches. It is advised to start with the less invasive home-use therapies and only expand to professional in-office treatments when the home-use treatments are not effective. When decided to continue with in-office treatments, again one should start with the least invasive ones.
Home-use products have several benefits including ease of use, convenience of self-application, and easier access but may require several weeks before taking effect. In-office treatments are generally more invasive and more effective under specific conditions and can provide instant relief, e.g., an adhesive sealing or restoration.
A remaining aspect is the placebo effect, which is an important and potentially beneficial side effect when dealing with pain and its treatment and management. Using arthritis of the knee as an example in the medical field, it has been impressively shown that sham endoscopic interventions lead to the same reduction of pain and symptoms as conventional treatment modalities [41]. In addition, prescription of differently colored pills resulted in significant differences in pain reduction [42]. Whereas a red placebo tablet, for instance, showed comparable pain relief as the best antirheumatic test pill used, the blue equivalent showed the least effect. Thus, improved psychological cotherapeutic strategies may one day become an important auxiliary aspect in dentin hypersensitivity management, especially when it comes to changing patients’ expectations of treatment outcomes and confidence. The psychological training of dental professionals still has some room for development.
15.6.1 At-Home Therapy
For home use, both toothpaste and mouthrinses are available. There are a few studies on chewing gum but the results are not very reliable [43, 44]. The working mechanisms fall under two basic categories, being nerve desensitization and occlusion of exposed dental tubules (Table 15.4).
Table 15.4
Active ingredients in products for at-home therapy
|
Calcium/hydroxyapatite carriers
|
||||||||
|
Active ingredient
|
Potassium
|
Strontium
|
Fluoride
|
Stannous salts
|
Oxalates
|
Calcium phospho silicate (Novamin)
|
Arginine calcium carbonate (Pro‐arginin)
|
Nanohydroxyapatite
|
|
Mechanism of action
|
Nerve Desensibilization
|
Precipitative occlusion of dentin tubules
|
||||||
|
Compatible with Fluoride
|
Yes
|
SrCl not
SrAc yes
|
Yes
|
Yes
|
Yes
|
MonoFluoro Phosphate (MFP)
|
MFP
Sodium Fluoride
|
Yes
Caveat: some pastes do not contain F
|
15.6.1.1 Nerve Desensitization
Potassium salts and, to a lesser extent, strontium and calcium [45] are agents that may have a direct desensitizing action on the nerves located at the pulpal side of the tubules. Therefore, the ions must be able to pass through the dentinal tubules against the dentin fluid flow and build up a sufficiently high concentration to desensitize the nerves at the interface of the inner dentin surface and the pulpal chamber. A concentration of 8 mM might be necessary needing a lag time of several weeks before pain relief is experienced. Once at the nerve site, potassium alters the cell’s electrical potential, resulting in depolarization, making the cell less responsive to stimuli. When people stop using the product, the potassium will diffuse away, and sensitivity reestablishes. Strontium as other divalent cation may operate by a different mechanism from potassium, such that the membrane of the nerve cell is stabilized but the potential of the cell remains unchanged [45].
Mainly potassium nitrate (5 %), citrate (5.5 %), and chloride (3.75 %) have been formulated into toothpastes as each of the salts provides 2 % potassium, which is needed for relief. In the United States of America, desensitizing toothpastes typically contain 5 % potassium nitrate, to meet FDA regulations. Many manufacturers have a potassium-based desensitizing product, suggesting it being (or having been) the “golden standard.” A recent Cochrane review included six studies in a meta-analysis, which showed a statistically significant effect of potassium nitrate toothpastes on air blast and tactile sensitivity tests at 6–8 weeks follow-up, respectively. The subjective reports of the patients on dentin hypersensitivity, in contrast, failed to show a significant effect at the respective time points (Table 15.5) [46].
Table 15.5
Results of a systemic review and meta-analysis on the effect of potassium-containing toothpastes on dentin hypersensitivity
|
Outcome and comparison
|
No. of studies
|
No. of participants
|
Effects size (95 % CI) (std mean differencea)
|
|---|---|---|---|
|
Tactile
|
5
|
1.19 (0.79, 1.59)
|
|
|
Potassium nitrate no F
Versus
No potassium nitrate no F
|
1
|
110
|
0.72 (0.33, 1.11)
|
|
Potassium nitrate plus F
Versus
No potassium nitrate plus F
|
4
|
246
|
1.34 (0.97, 1.71)
|
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


