Dietary factorsa
Chemical factors
Citrus juices and other acidic fruit juices
pH
Acidic (un-)carbonated beverages
Buffering capacity
Acidic sport drinks
Type of acid (pKa values)
Citrus fruits and other acidic fruits and berries
Surface adhesion properties
Salad dressing
Calcium concentration
Vinegar conserves
Phosphate concentration
Wines
Chelating properties
Acidic fruit-flavored candies
Acidic chewing gums
Cider
Acidic herbal teas
Alcoholic mixed drinks
4.2.1 Beverages
4.2.1.1 Beverages Consumption Trends
When discussing dental erosion and dietary factors, much of the focus has been given to acidic beverages, especially soft drinks. A survey on the food intake trends by US adolescents showed that the consumption of soft drinks had increased considerably (up to about 300 %) between 1965 and 1996 [3], exposing adolescents to potentially erosive conditions, and increasing their risk of developing dental erosion. This change in dietary pattern was driven in the USA by the increased popularity of cola drinks in the 1980s, fueled by massive marketing strategies imposed by large soft-drink companies. Supporting this trend, the drink serving sizes tripled from the 1950s to late 1990s (6.6–20 oz), when soft drinks consumption seemed to have reached its peak.
From the late 1990s to nowadays, soft drinks (specially sugary ones) started to be associated with major US health problems, such as poor diet quality, weight gain, obesity, and, in adults, type 2 diabetes [4]. It has also affected dental health as they have been related to increased risk for dental caries [5] and dental erosion. Effort has been made by governments around the world to limit the availability of soft drinks in schools, which is one of the main channels that encourage greater consumption of soft drinks. More than 30 national and subnational governmental bodies have made efforts to restrict availability, and the soft-drinks industry has also taken some limited voluntary action [6]. Coincidently or not, the overall consumption of beverages has decreased from 54 gal (204 l)/person/year in 1998 to 44 gal (166 l), in 2013 (according to Beverage Digest; http://www.cbsnews.com/news/americans-rekindle-love-for-drinking-water/).
Interestingly, some reports have suggested that water has partially replaced soft drinks, as the consumption of bottled water has increased in the same period [7]. This general trend, however, may not apply to all types of beverages, as the consumption of diet soft drinks, for example, has slightly increased (3.4 and 5.1 %, for US males and females, respectively) from 2000 to 2010 [8]. Since the erosive potential of diet and regular soft drinks has shown to be comparable in laboratorial testing [9], there is no clear advantage in this change of dietary pattern, from the dental erosion perspective. Additionally, we have witnessed a substantial increase in the popularity and availability of other types of beverages, including flavored/enhanced water, sports drinks, energy drinks, and fruit juices, most of them with comparative or higher erosive potential. Therefore, despite the soft-drink consumption having reached a plateau or even declined in the USA, it is safe to assume that the population is still widely exposed to acidic beverages that are potentially erosive.
A general overview of the current beverage consumption worldwide shows great variation among countries (http://a.tiles.mapbox.com/v3/slate.soda.html; source: Euromonitor International, 2011). The increased consumption of acidic beverages observed in the USA over the last decades is comparable to other developed countries and may serve as projection for developing countries recently experiencing changes in their dietary habits. Epidemiological studies have shown increase in dental erosion prevalence and some of them have related this fact with the consumption of acidic beverages (Table 4.2). In summary, while beverage consumption has plateaued in some developed countries, there is still an overall trend for increase in soft-drink consumption worldwide, which was projected to grow from just under 83 l/person per year, in 2007 (equivalent to a total of 552 billion liters), to a 95 l/person per year, in 2012 [39].
Table 4.2
Clinical evidence linking acidic beverages and dental erosion
|
Authors
|
Year
|
Country
|
Age
|
Sample
|
|---|---|---|---|---|
|
Significant association
|
||||
|
Lussi et al. [10]
|
1991
|
Switzerland
|
26–30; 46–50
|
391
|
|
Millward et al. [11]
|
1994
|
UK
|
12–14
|
101
|
|
Johansson et al. [12]
|
1997
|
Saudi Arabia
|
19–25
|
95
|
|
O’Sullivan and Curzon [13]
|
2000
|
UK
|
3–16
|
309
|
|
Moazzez et al. [14]
|
2000
|
UK
|
10–16
|
21
|
|
Al-Malik et al. [15]
|
2001
|
Saudi Arabia
|
2–5
|
987
|
|
Harding et al. [16]
|
2003
|
Ireland
|
5
|
202
|
|
Dugmore and Rock [17]
|
2004
|
UK
|
12–14
|
1149
|
|
Luo et al. [18]
|
2005
|
China
|
3–5
|
1949
|
|
El Karim et al. [19]
|
2007
|
Sudan
|
12–14
|
157
|
|
Mungia et al. [20]
|
2009
|
USA
|
12–17
|
307
|
|
Sanhouri et al. [21]
|
2010
|
Sudan
|
12–14
|
1138
|
|
Murakami et al. [22]
|
2011
|
Brazil
|
3–4
|
967
|
|
Okunseri et al. [23]
|
2011
|
USA
|
13–19
|
1314
|
|
Aidi et al. [24]
|
2011
|
Netherlands
|
10–12
|
572
|
|
Huew et al. [25]
|
2011
|
Lybia
|
12
|
791
|
|
Bartlett et al. [26]
|
2011
|
UK
|
18–30
|
1010
|
|
Mulic et al. [27]
|
2012
|
Norway
|
18
|
1456
|
|
Nayak et al. [28]
|
2012
|
India
|
5
|
1002
|
|
Chrysanthakopoulos [29]
|
2012
|
Greece
|
13–16
|
770
|
|
Hamasha et al. [30]
|
2014
|
Jordan
|
12–14
|
3812
|
|
No association
|
||||
|
Bartlett et al. [31]
|
1998
|
UK
|
11–14
|
210
|
|
Williams et al. [32]
|
1999
|
UK
|
14
|
525
|
|
Deery et al. [33]
|
2000
|
USA, UK
|
11–13
|
129 (USA); 125 (UK)
|
|
Milosevic et al. [34]
|
2004
|
UK
|
14
|
2385
|
|
Wiegand et al. [35]
|
2006
|
Germany
|
2–7
|
463
|
|
Gurgel et al. [36]
|
2011
|
Brazil
|
12–16
|
414
|
|
Manaf et al. [37]
|
2012
|
Malaysia
|
19–24
|
150
|
|
Aguiar et al. [38]
|
2014
|
Brazil
|
15–19
|
675
|
4.2.1.2 Beverages Involvement in Dental Erosion
Reflecting the high consumption of acidic beverages, the number of clinical studies investigating their relationship with dental erosion has increased in recent years. Although most studies have shown this association, some have failed as can be seen in Table 4.2. The multi-factorial nature of erosion makes it difficult to identify single etiological factors, such as acidic beverages. Other important factors may be the limited relevance of the dietary patterns at the time of collection compared to those when tooth erosion actually occurred [37]. This is confounded by the relatively slow progression of erosive lesions and the difficulty in diagnosing the different stages of lesion development clinically. Nonetheless, an epidemiological study showed that between 56 and 85 % of children at school in the USA consumed at least one soft drink daily, with the highest amounts ingested by adolescent males. Of this group, 20 % consumed four or more servings daily [40]. These results are disturbing, since it has been shown that any consumption of carbonated beverages increased the chances of dental erosion by 59 %, while drinking four or more glasses per day resulted in a 252 % increase [17].
A comprehensive evaluation of existing clinical studies has showed that soft drinks were associated with about 2.4-fold risk increase of dental erosion, despite the only moderate number of total studies available and the relatively small number of subjects [41]. Clinical studies in the area provide the most direct evidence that frequent exposure to acidic beverages can result in dental erosion. Based on the clinical data mentioned in Table 4.2, acidic fruits and juices, carbonated and uncarbonated beverages, sports drinks have been associated with causing erosion. Experimental clinical studies involving intraoral pH measurements after drinking or rinsing with acidic beverages (ciders, citric fruit juices, fruit juice drinks, flavored drinks, diet drinks) indicated that most acidic beverages only cause transient lowering of the pH of oral fluids [42]. Unusual or excessive consumption of specific dietary substances such as lemon juice, orange juice, carbonated cola beverage, orange cordial, and fruit-flavored drinks have been implicated based on case reports [42]. Figures 4.1, 4.2, and 4.3 illustrate cases of patients suffering from dental erosion at different degrees of severity. All cases are associated to frequent consumption of carbonated beverages.
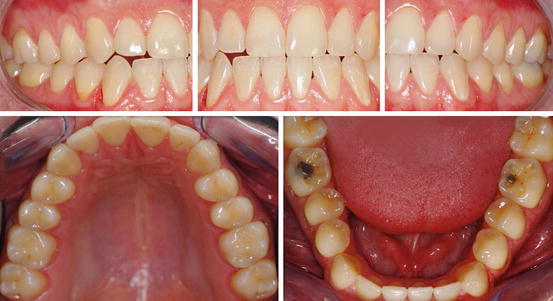
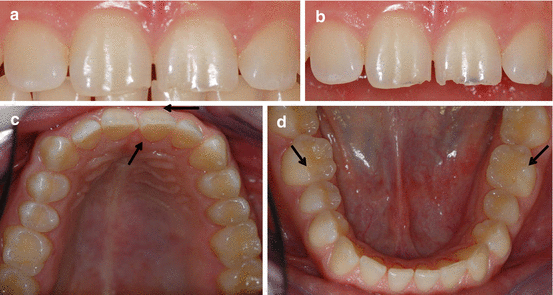
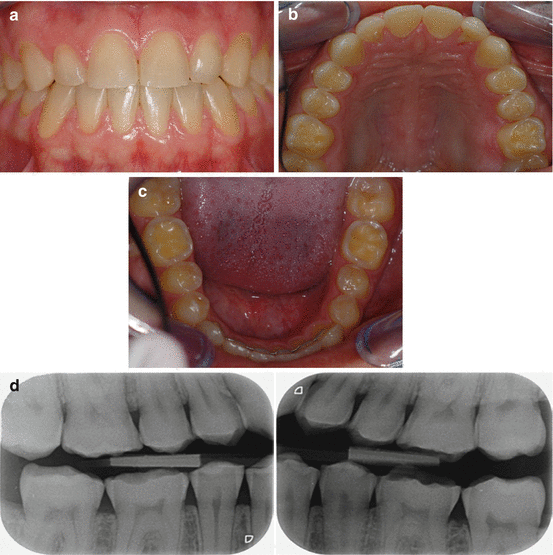

Fig. 4.1
Male 24 years old. Frequent consumption of soft drinks. Early signs of the erosion process may be identified in the whole mouth. Absence of the perikymata pattern and shiny feature of the enamel on facial aspect of maxillary and mandible teeth. Rounding of marginal ridges and cusps on occlusal surfaces of permanent molars. On occlusal surfaces of teeth 36 and 46 observe cupping and restored areas that appear at a higher level than the surrounding dental tissue

Fig. 4.2
Male 18 years old. Frequent consumption of soft drinks: (a) Established erosive lesions on teeth 11 and 21 are masked when the patient occludes. (b) Erosive bluish shadow lesions at the incisal edges of teeth 11 and 21. (c) Facial and lingual erosion with a thin enamel band at the gingival margin (arrows). (d) Slight cupping on teeth 36 and 46 (arrows)

Fig. 4.3
Male 33 years old. Frequent consumption of soft drinks: (a) More advanced erosive lesions with dull appearance of front teeth. (b) Lingual erosive lesions on maxillary anterior teeth. The groove-fossa-system is no longer identified on occlusal surfaces of posterior teeth. (c) Localized exposure of the dentin on occlusal surfaces of permanent first molars. (d) Bitewing radiographs show flat eroded occlusal surfaces
Of limited clinical relevance, animal models [43–48] and in vitro studies [49, 50] have also been used to evaluate the erosive potential of different foods and beverages. These studies present the ability to simulate specific clinical conditions permitting comparison of large numbers of foods and beverages under well-controlled conditions. Yet, translation of the findings to the clinical reality is confounded by differences in experimental design and methodology among the studies and concerns over their clinical relevance [2]. Despite these limitations, acidic beverages that have been identified in clinical studies as etiologic factors in erosion have been confirmed in animal and in vitro studies.
4.2.1.3 Evaluation of Erosive Potential of Beverages
Although extensive data can be found on beverage consumption, it should be borne in mind that they can greatly differ on their erosive potential. Beverages can be classified into nine broad groups: water (bottled or tap), milk (including flavored), fruit juice (100 %), soda/soft drinks (regular and diet), fruit drinks, sports/energy drinks, coffee, tea, and alcoholic beverages [51]. According to Lussi et al. [39], soft drinks are mainly composed of filtered water, artificial additives, and refined sugar; sports drinks, which are designed to replenish fluids lost during activity, typically contain water, electrolytes, and sugar; and, energy drinks are basically soft drinks that contain some forms of vitamins, caffeine, and other chemicals that boost energy for a very short span. Of interest for dental erosion are those beverages that present lower pH and higher buffering capacity, which includes hundreds of commercially available options. Their erosive potential varies even within each group, depending on brands and even geographic location. For instance, the same beverage formulation was reported to present distinctive erosive potentials because of the different calcium and fluoride contents in the local water supplies used to produce them [9, 52]. Differences in taste or flavor can also affect the erosive potential of drinks and foods, with fruit or other acidic flavorings leading to lower acidity and higher erosive potential [39]. A study comparing different solutions of tartaric, malic, lactic, ascorbic, phosphoric, and citric acids with concentrations to equal their sensorial acidic taste showed that they present different erosive potentials [53]. Some physical characteristics of the beverages, such as increased adhesiveness and displacement, may increase their contact time with the tooth surface [54–56], increasing the erosive potential as well.
Due to the high number of beverages in existence, it is not feasible to conduct clinical test to obtain the erosive effect of all available beverage products, thus one should rely on their erosive potential for the recommendation of preventive measures for dental erosion. The erosive potential of beverages can be determined more easily and faster by laboratorial screening tests [57], which generally consider the pH, buffer capacity (titratable acidity), degree of saturation, calcium and phosphate concentration, and the presence of potential erosion inhibitors. pH has been described as one of the most determinant factor associated with dental erosion [57]; although it is crucial to understand that other properties and characteristics, as well as clinical application conditions including acid volume, frequency, and time of exposure [58, 59] can also affect the erosive potential. As example, calcium-enriched orange juice presents low pH (3.8), but has no erosive potential due to its high calcium content [60]. Titratable acidity, a property that shows how strong the acid is counteracting the protective effects of saliva [61], is also considered an important predictor [62], especially when the solution stays in contact with the tooth surface and is not rapidly cleared by saliva.
Larsen [63] suggested that erosion potential could be calculated based on the degree of saturation with respect to both hydroxyapatite and fluorapatite, by determining the pH, calcium, phosphate, and fluoride content of a beverage. Using a more elaborated approach, Lussi et al. [39, 64] developed an erosion prediction model including some of the beverage properties and characteristics. This model has shown good correlation with the ability of the beverages to soften the surface of enamel. For a more dynamic evaluation of the erosive potential of beverages, a laboratorial method called pH-stat has been used [65]. The pH-stat is based on the reaction between H+ and the test substrate, usually hydroxyapatite (HAp) crystals, and measures the dissolution rate of HAp, at a constant pH determined by the test beverage. Since the dissolution of HAp consumes H+, the rate/volume at which a titrant (acid) is added to maintain the pH of the test beverage is proportional to the rate of HAp dissolution [66], defining its erosive potential. Table 4.3 shows the different erosive potential of acidic beverages, based on the pH-stat test, compared to citric acid (most erosive) and drinking water (not erosive).
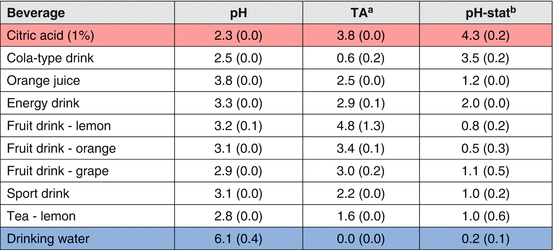
Table 4.3
Chemical properties of beverages (mean and standard-deviation) determining their erosive potential

These screening tests are valuable providing data on the erosive potential of acidic beverages, in a relatively fast and inexpensive manner. Determination of the erosive effect will depend on many other biological and behavioral variables and can only be achieved in more clinically relevant experimental conditions. Nevertheless, the erosive potential is an important parameter that can be used for patient counseling.
4.2.1.4 Modification of Erosive Potential of Beverages
While patient’s behavioral changes targeting the reduction of acidic beverages consumption are probably the most logical recommendation for those suffering from dental erosion, it should be borne in mind that patients may not necessarily comply with those measures. In this scenario, consumption of less erosive alternatives may be considered. Modifications on any of the relevant properties mentioned above (pH, buffer capacity, degree of saturation, calcium and phosphate concentration) may lessen the erosive potential of beverages. The addition of calcium and/or phosphate ions to beverages decreases the driving force for dental surface dissolution, as they become saturated with respect to the tooth minerals [67]. Commercially available acidic beverages have been supplemented with calcium in order to compensate for the deficient calcium intake, aiming to prevent some general health conditions, such as osteoporosis. As a positive side effect, these beverages present lower or non-erosive effect. Some studies have shown enamel demineralization reduction or inhibition for Ca-containing drinks when compared to those without Ca [58, 60, 68, 69]. Nowadays, several calcium-containing beverages are available in the market and they have shown reduced erosive potential [59]. Experimental combinations of Ca with other ions (Fe, P, and F) [70, 71] and proteins like ovalbumin [72] have also shown some reduction on erosive potential. Table 4.4 shows some examples of supplemented beverages and how their erosive potential is reduced, as measured by pH-stat.
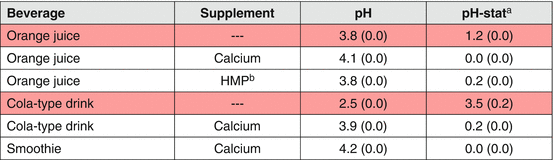
Table 4.4
Erosive potential of supplemented commercial beverages

Higher amounts of calcium seem to provide the best erosive potential reduction. However, it can also change the taste and the stability of the drink [67]. Another potential negative side effect is that excessively higher amounts may exceed the tolerable upper limit (level that may cause adverse health effects) of calcium (60 mmol/day), increasing the risk of kidney stones and of interference in the absorption of other minerals, including zinc, magnesium, and phosphorus [73]. Therefore, lower and effective concentrations are preferred. There is also some speculation on the influence of the type of calcium compound used. For instance, calcium lactate pentahydrate has lactate anions that can contribute to its anti-erosive effects, since it forms stable complexes with calcium [74] that appears to be just strong enough to protect the calcium ions from binding to other more stable complexing compounds present in the juice and thus allowing them to be available to interact with the tooth surface [65].
Casein phosphopeptide-stabilized amorphous calcium phosphate (CPP-ACP, Recaldent) has also been explored in laboratorial testing, as beverage additive in sport drinks [75] and citrus-flavored soft drinks (carbonated or not) [76]. The CPP-ACP-modified beverages presented reduced or no erosive potential. The likely mechanism has been described to be related to the increased availability of calcium and phosphate ions at the enamel surface, as well as the formation of CPP-ACP nanocomplexes on the enamel surface, reducing the possible sites for enamel dissolution [76]. Similarly, experimentation with nano-sized hydroxyapatite has been done. Although hydroxyapatite presents undesirable lower solubility than other calcium compounds, the use of nanoparticles seems to allow for higher reactivity and release of calcium and phosphorus [77, 78]. Reduction on the erosive potential of a sport drink has been observed, but pH changes, precipitation and possible changes in taste of the modified drink have also been reported [77], and need to be further investigated.
Contrary to calcium, supplementation with inorganic Pi has not shown to offer relevant reduction in the erosive potential of beverages. According to Lussi et al. [39], at the pH of erosive drinks (approximately 2–4), only a minute fraction of the total Pi is in the form of PO4 −3 ion, which is the only important Pi species in the ion activity product of HAP and FAP. As a result, large quantities of Pi would be required to raise the degree of saturation of the acidic solution, in order to see an anti-erosive effect. Foods and beverages are more likely not to present such high levels of Pi.
The literature is contradictory with regard to the erosive potential of acidic drinks and foods containing fluoride. Some laboratorial studies have shown that fluoride addition to beverages, in concentrations excluding toxicologically side effects, is incapable of preventing erosion, as demonstrated testing 18 soft drinks [79]. In drinks with pH above 3, F reduced the in vitro development of erosion by 28 %; while in drinks with pH below 3, erosion was not affected, despite total F concentrations of 20 parts per million and saturation with calcium fluoride [80]. However, some other studies have shown that the erosive capacity of different drinks was significantly and negatively associated with their F concentration [39, 64, 81]. Methodological differences may have contributed to these conflicting results. It is important to point out, however, that fluorides have been important in erosion prevention, when used in different vehicles (toothpaste, mouthrinses, varnish), at different concentrations, as presented in Chaps. 8 and 9.
Supplementation with food polymers has also been investigated and they have shown ability to reduce erosion due to their possible adsorption to the dental surfaces, leading to the formation of an acid-protective layer. This layer could reduce the exchange of H+ and of calcium and phosphate ions between the hydroxyapatite and the solution [66]. Their protective properties, however, seem to be related to the type of polymer and experimental conditions. Polyphosphates of relatively longer chain length, such as sodium hexametaphosphate, seem to be more capable of reducing dental erosion than others, like tripolyphosphates and pyrophosphate. They also showed enhanced protection against erosion when used in association to calcium [65]. However, its clinical benefit is still unclear, as evidence for protection can be controversial [60, 82], thus requiring further investigation. Similarly, surface-protective effect has been reported to ovalbumin [72]. However, contrasting results were observed for xanthan gum, another food additive [65, 66]. Probably, the protective effect of the gum is minimal and its role as an additive of acid drinks might be more related to the improvement in the acceptability of the calcium-modified drinks than as an anti-erosive agent [65].
Slight modifications in taste, either by the additives themselves or by taste masking agents may make these alternatives less attractive to some individuals. However, in a brief taste evaluation of modified orange juices, only some tasters reported differences between the test juice (with calcium and polyphosphate) and the original, and most of them considered the taste to be acceptable [60]. Dietary supplements as effervescent tablet containing calcium and an acid ⁄base regulating powder were able to reduce the erosive potential of orange juice, without noticeable changes in taste [69]. Although these observations are encouraging, it is still necessary to investigate other combinations of beverages and types and amounts of additives. Despite being a viable and promising alternative, beverage modification should not be seen as the only preventive measure for dental erosion since it is chemically impossible to modify all erosive solutions [83]. Instead, it should be considered as an additional preventive measure for the management of dental erosion.
4.2.2 Other Acidic Foods
In addition to beverages, other acidic foods have also been associated to dental erosion, with some clinical evidence for the consumption of vinegar and vinegar conserves, citric fruits, acidic berries, and other fruits (apple, pears, and plums) [10, 26, 84–86]. Acidic vegetables, a factor not always taken into account in clinical studies, have also shown to be associated to erosion, although their erosive effect may be confounded with the individual’s overall preference for acidic foodstuff [87].
4.2.3 Acidic Candies
Acidic candies have been reported to be significant factors on erosion development. Although strong clinical evidence is not available, there are some reports and simulation studies supporting their erosive potential. They contain organic acids such as citric acid and malic acid to develop the characteristic sour flavor [88]. Despite the saliva-protective factors, sucking on sour candies can reduce the salivary pH levels below to the critical value for dental demineralization, therefore posing a risk for erosion of dental surfaces [58, 89]. This has been demonstrated in laboratory [45] and clinical [27, 30, 58, 90] settings. Placing acidified candies immediately next to the tooth surfaces can result in a concentrated solution of the dissolved candy with low pH, which has long been considered as a risk factor for both tooth surface softening and loss [91].
Solid or hard candies, such as lollipops and “jawbreakers” [88, 92], are normally difficult to bite, and therefore usually consumed by sucking or licking. As they dissolve slowly, they can be kept in the mouth for extended periods of time. This allows for prolonged and continuous exposure of the teeth to acids. Jawbreakers are mostly consumed by children with some even competing with each other to keep the candy in the mouth for longest period [88]. The flavor is influenced by the type of acid and concentration used; therefore, it is expected that different flavors may result in different erosive potentials. It has been shown that sour flavors present higher erosive potential compared to original flavors [93]. Similarly, the size of the candy is an important aspect, since it will determine the total length of exposure to the erosive challenge. Another type of acidic candy, the so-called candy spray, is sprayed directly into the mouth creating an immediate sour-fresh taste and tingling feeling on the tongue [89]. It has been reported to present high erosive potential due to low pH and high buffering capacity. Severe dental erosion has been reported to be associated with the extensive consumption of this candy [89].
Acidic candies may pose a high risk for erosion in particular populations. It primarily affects children and adolescents, populations vulnerable to uncontrolled and excessive high consumption behaviors. It has been reported that 70 % of parents were unaware that their children were consuming a type of acidic candy [89]. Acidic candies are also problematic for patients suffering from dry mouth, such as those receiving head and neck irradiation therapy, as they often present low salivary flow rates and buffer capacity [94]. These patients may experience natural dietary changes toward a higher intake of acidic saliva stimulating food, including acidic candies and lozenges [94]. Although some benefits are derived from the salivary flow stimulation, it has been observed that after consumption of acidic hard-boiled candy (with tartaric acid and rhubarb flavor), saliva became significantly more undersaturated with respect to tooth minerals and failed to return to clinically normal values, as observed in healthy individuals [94]. Counseling should therefore be provided for these high-risk populations, regarding the selection and consumption of acidic candies.
Similar to beverages, adding anti-erosion agents, such as calcium, in acidic hard candies has also been considered. Both candies with and without calcium were able to stimulate saliva secretion, with the former releasing higher amounts of calcium into saliva. This translated into lower ability to demineralize tooth minerals, which indicates reduced erosive potential [95].
4.2.4 Chewing Gum
Chewing gums have been promoted by dental professionals as beneficial for the dentition, because of their ability to increase the flow rate and pH of saliva, potentially clearing and neutralizing acids and promoting enamel remineralization [96]. In addition, gums can also act as vehicles for a sustained delivery of some therapeutic agents for caries prevention [97]. Sugar-free chewing gums are, therefore, considered important for the management of dental caries. However, it has been speculated that frequent use of some of the acidic chewing gums may present potential for dental erosion development, especially on the occlusal surfaces of posterior teeth [98]. In the first minutes after chewing on strawberry-flavored acidic gums, the pH of saliva was observed to be reduced to 3.98, a level lower than the critical for tooth demineralization, while no pH drop was observed for the peppermint-flavored gum [98]. Although this is followed by a rise in pH to normal levels in the subsequent minutes, there is a potential risk that repeated and rapid replacement of gum with a fresh piece, commonly done once the flavor of the gum is lost, may keep the low pH values at tooth surfaces for longer, increasing the risk for dental erosion. For instance, replacement of gum every 4 min was able to cause significant erosive tooth wear on dentin [98]. More clinical evidence is needed to better understand the importance of acidic gums on erosion.
4.3 Medications
The frequent use of acidic medications that come in direct contact with teeth has been identified as an etiologic factor in dental erosion. Generally, acids function as buffering agents contributing to chemical stability, physiological compatibility, drug dispersion, and flavor improvement [99]. Acetylsalicylic acid (aspirin) [100], liquid hydrochloric acid [101], ascorbic acid (vitamin C) [102], iron tonics [103], cocaine [104], acidic oral hygiene products [105] or products with calcium chelators [106], and acidic saliva substitutes [101], salivary flow stimulants [106], and hospital mouth-cleaning aids [107] have been implicated in dental erosion based on case reports and/or laboratory studies. Some of the more relevant findings relating medications to erosion are described as follows.
4.3.1 Analgesic
Many soluble analgesic preparations contain citric acid. Studies have shown that they present erosive potential [108, 109] and case reports suggest that their excessive use can lead to dental erosion [110, 111]. A laboratory test of six commercial brands of analgesics available in the UK showed that they present different erosive potentials. Although most of them were deemed not to be harmful to enamel, one brand was considered to be potentially erosive even in clinical conditions [109]. This analgesic in particular (Aspro™), presented the highest titratable acidity, the lowest calcium concentration, and no detectable phosphate. Further clinical testing was recommended to better understand and confirm the erosive potential observed. In another study, a buffered (500 mg acetylsalicylic acid, 300 mg calcium carbonate) acetylsalicylic acid chewable tablet showed no changes in the enamel surface structure after laboratory simulation exposure times of 1, 5 and 60 min. In contrast, enamel erosion was observed for an unbuffered acetylsalicylic acid chewable tablet even after 1 min of exposures, which was accentuated after 5 and 60 min [108]. Chewing increases the contact time between the tablet and tooth surfaces, considerably enhancing the risk for erosion. This was observed in a study comparing children with juvenile rheumatoid that were receiving large doses of aspirin in one of two forms, swallowing or chewing, at the time of the study. All children who were chewing the aspirin (25 out of 42) developed severe erosion on their maxillary and mandibular primary molars and first permanent molars. None of the children who swallowed aspirin tablets (17 out of 42) developed any eroded areas [100]. The authors concluded that aspirin was the reason for the erosive lesions. Considering the existing case reports and the reported erosive potential of some analgesics, dentists should advise patients who habitually use soluble/chewable analgesics for chronic conditions to select analgesic options with no or minimal erosive potential.
4.3.2 Vitamins
Reported vitamin consumption showed to be associated with dental erosion progression [87]; however, there was no further discrimination into chewable vitamins and vitamins that are swallowed. The authors suggested that the use of vitamins may represent a lifestyle that increases the chance of erosive wear to progress. There is some evidence associating chewable vitamin C (ascorbic acid) intake with erosion development [86, 112, 113]. This finding was also substantiated by a meta-analysis study investigating dietary factors and dental erosion [41].
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


