Fig. 28.1
Brännström presumed that stimuli move fluid in or out of dentin and that this fluid activates intradental or pulpal nerves to cause pain
28.1.5 Etiology
The most important factor in the etiology of dentin hypersensitivity is exposed dentin as a result of loss of enamel associated with tooth wear or trauma and/or as a result of gingival recession associated with exposure of root surfaces [13]. Tooth wear refers to the irreversible loss of tooth structure and includes conditions such as abrasion, erosion, attrition, and abfraction. Occurrence of wedge-shaped cervical lesions is often associated with abrasion and occlusal hyperfunction. Although there are many causes for non-carious cervical lesions of dentin, improper brushing is considered one of the major causes.
28.1.6 Prevalence
The prevalence of dentin hypersensitivity has been reported over the years in a variety of ways. Dentin hypersensitivity is a common condition with a reported prevalence between 4 and 69 % in the adult population [14]. Another research reported the prevalence of dentin hypersensitivity varies, but averages about 57 % and peaks between 20 to 40 years of age [15]. It has been reported that more than 40 million people in the U.S. are affected, 14.3 % of all dental patients, between 8 and 57 % of adult dentate population, and up to 30 % of adults at some time during their lifetime [16]. Among periodontal patients, the prevalence is even higher (60–98 %). Dentinal hypersensitivity occurs with a first peak in 20 to 30 year olds and then with another peak later in the 50s. The condition involves mainly the facial cervical surfaces of teeth and is mostly found in premolars and canines [17, 18]. Patients who have received periodontal treatment are particularly sensitive to this condition because of the loss of cementum following periodontal therapy. In addition periodontal disease and improper brushing can also cause gingival recession accompanied by sensitive teeth [17]. Dentinal hypersensitivity has been researched extensively through the years and many authors express an agreement that dentinal hypersensitivity is either under-reported by the dental patient population or misdiagnosed [19].
28.1.7 Treatment
Logical treatment regimens attempt (1) to occlude dentin tubules or (2) to block the pulpal nerve activity by increasing the potassium ion concentration, typically with potassium nitrate or potassium chloride. A variety of methods with tubule blocking agents is available for management of dentinal hypersensitivity comprising resins, glass-ionomers, primers, dentin adhesives, protein precipitants, oxalates and laser treatment [20–24]. According to a recent survey in the USA, 45 % of dental practitioners regularly use oxalates and approximately 60 % use glutaraldehyde/HEMA as topically applied agents to treat dentinal hypersensitivity [21, 25]. Although the mechanisms of pain transmission across dentin are not fully understood, both dentin permeability and hypersensitivity are reduced when the dentinal tubules are occluded. Therefore, hypersensitivity treatment strategies have mainly focused on tubular occlusion.
28.1.8 Permeability
According to the hydrodynamic theory, dentinal hypersensitivity is related to the movement of intertubular fluid. Several studies have demonstrated the relationship between open tubules on the exposed cervical surface and hypersensitivity [12, 26]. In vitro studies investigating dentin desensitizers have focused on dentin permeability and hydraulic conductance as measures for effectiveness of these agents.
One of the laboratory methods that has been frequently used and that focuses directly on dentin fluid flow is a dentin disc model for assessment of permeability and hydraulic conductance [12, 27]. This model or modifications thereof have been used to assess both professional desensitizers and dentin adhesives [28–32], and the method is commonly considered a good and reliable model for in vitro screening and testing of the potential of desensitizing agents [33].
Ishihata et al. [34] have designed a modified split-chamber device using a chemiluminescence reaction to evaluate the liquid permeability of dentin discs. This test is also considered a suitable and reliable screening method for assessment of topical desensitizing agents efficacy in reducing or eliminating dentin permeability, irrespective of the tubular blocking mechanism used [35].
28.1.9 Clinical Trials
Clinical trials on dentin hypersensitivity should as a rule use randomized group assignments, be double-masked and contain a placebo product that is identical to the test product except that it does not contain the active ingredient [36, 37]. It is critical to evaluate the placebo effect, which can be very strong in such studies. Conclusions derived from early studies on dentin hypersensitivity using single-masked methods, or inappropriate stimuli should be viewed with caution.
Limited evidence indicates that tooth brushing without toothpaste decreases hypersensitivity scores while brushing with toothpaste increases dentin hypersensitivity scores unless the toothpaste contains a potassium-containing desensitizing agent. Although a recent meta-analysis of six clinical trials using potassium-containing desensitizing toothpaste demonstrated reductions in the symptoms of patients’ dentin hypersensitivity compared to control toothpaste [38], the scientific evidence supporting the use of potassium salts to reduce nerve activity is based mostly on in vivo animal studies and one recent human in vivo trial [39].
28.1.10 New Approach
Desensitizing products may be in the form of topically applied agents such as resins, primers, dentin bonding agents and others [40–43]. Recently there has been an increasing interest in calciumphosphate-containing materials [34, 44–48]. Such calcium phosphate-containing materials are potentially transformed to hydroxyapatite as a final product, which is the principal mineral in teeth. The proximity of hydroxyapatite to the natural tooth structure and its biocompatibility makes these materials useful in a variety of dental applications. The development of the next generation calcium phosphate cement materials is expected to have greater efficacy in a wide range of clinical applications.
A spin-off from calcium-phosphate cement developments is a topically applied desensitizer, claimed to precipitate hydroxyapatite as biocompatible mineral on dentin and inside the openings of dentinal tubules. This material showed outstanding characteristics in dentinal tubule occlusion and favorable reduction in dentin permeability (Fig. 28.2).
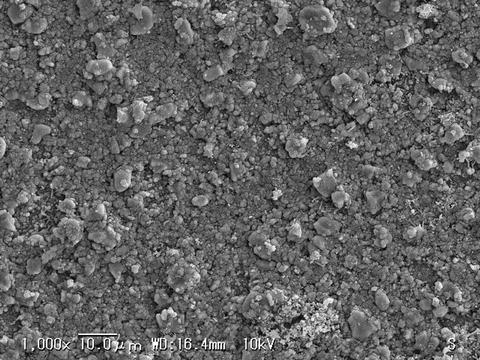

Fig. 28.2
SEM photograph of dentin surface after application of calcium-phosphate containing compound. A layer of precipitated crystals (hydroxyapatite and other apatite species) covered the dentin surface and occluded the dentinal tubules
Human saliva contains an abundance of calcium and phosphate ions too. The supersaturation of salivary fluid is expected to contribute to further apposition and growth in size of hydroxyapatite crystals formed in the oral environment [49].
Petrou described a breakthrough technology based upon arginine and calcium carbonate that provides clinically proven benefits with respect to rapid and lasting relief of dentin hypersensitivity [50]. Arginine and calcium are found naturally in saliva, and they work together to accelerate the natural mechanisms of occlusion to deposit a dentin-like mineral, containing calcium and phosphate, within the dentin tubules and in a protective layer on the dentin surface. Sodium calcium phosphosilicate has been shown in laboratory studies to rapidly occlude dentin tubules through the deposition of particles that react to form a protective layer on the dentin surface [51]. This material was originally developed as a bone regenerative material and is highly biocompatible.
Preliminary studies have shown that topical application of a combination of amorphous calcium phosphate (ACP) and casein phosphopeptide (CPP) can cause blockage of dentin tubules [52].
In this way the search for a natural desensitizing agent has led to the observation that calcium phosphate minerals obstruct dentinal tubule orifices mimicking the natural process of sclerosis.
28.2 Conclusion
Dentinal hypersensitivity is a common and significant dental problem with the symptoms, measurement and oral factors that contribute to dentinal hypersensitivity having been well characterized. Several theories have been developed to explain the mechanisms with respect to the structure of the dentin and pulp. This has lead to the development of treatments that may be permanent and non-invasive in nature.
Future treatment modalities for dentin hypersensitivity are currently under development that might combine the benefits of being both non-invasive and permanent yet cost effective for both dentist and patients.
The authors acknowledge gratefully the support of Professor W.J. Finger (Tohoku University) and his valuable advice for editing of this manuscript.
Open Access This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
1.
Canadian Advisory Board on dentin hypersensitivity. Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J Can Dent Assoc. 2003;69:221–6.
2.
Berman LH. Dentinal sensation and hypersensitivity. A review of mechanisms and treatment alternatives. J Periodontol. 1985;6:216–22.CrossRef
3.
Brännström M. A hydrodynamic mechanism in the transmission of pain producing stimuli through the dentine. In: Andersen DJ, editor. Sensory mechanisms in dentine. Oxford: Pergamon; 1963. p. 73–9.
4.
Schmidlin PR, Sahrmann P. Current management of dentin hypersensitivity. Clin Oral Investig. 2013;17(Suppl1):55–9.PubMedCentral
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


