The principal reason for providing periodontal therapy is to achieve periodontal health and retain the dentition. Patients with a history of periodontitis represent a unique group of individuals who previously succumbed to a bacterial challenge. Therefore, it is important to address the management and survival rate of implants in these patients. Systematic reviews often are cited in this article, because they provide a high level of evidence and facilitate reviewing a vast amount of information in a succinct manner.
The principal reason for providing periodontal therapy is to achieve periodontal health and retain the dentition. This objective includes restitution of form and function, esthetics, and avoidance of further periodontal disease progression. Although not mandatory to sustain life, many people prefer to have a full or functioning dentition. Replacement of lost teeth with conventional prostheses on natural teeth or with dental implants is desirable. In this regard, osseointegration of dental implants is a predictable treatment modality and an integral aspect of treatment planning in the periodontal patient who has or is expected to lose teeth. Patients with a history of periodontitis, however, represent a unique group of individuals who previously succumbed to a bacterial challenge. Therefore, it was deemed important to address the management and survival rate of implants in these patients. Systematic reviews often are cited in this article, because they provide a high level of evidence and facilitate reviewing a vast amount of information in a succinct manner.
Major causes of tooth loss in patients
It has been questioned whether dental caries or periodontal diseases are the main cause of tooth loss. Several investigations have indicated the main reason for tooth extraction in all age groups is caries ; however, others suggest it is periodontal diseases. An apparent interpretation of their findings is that caries causes tooth loss in more patients, but periodontal diseases are responsible for more teeth being removed in individual patients. In addition, periodontitis is the principal reason for edentulism in individuals vulnerable to periodontal diseases. Because more teeth are lost due to periodontal disease than any other oral affliction, the issue as to the success of placing dental implants in patients with a history of periodontitis is an important treatment planning consideration.
Etiologic agents of periodontitis and peri-implantitis
Bacteria are the main etiologic agents that induce periodontitis and peri-implantitis ; therefore, knowledge concerning the main pathogens is important for understanding the linkage between retention of implants and a patient’s history of periodontitis. Within the human oral cavity, hundreds of species of bacteria have been identified. In individuals with chronic periodontitis, it has been reported that the predominant bacterial species are gram-negative anaerobes; however, other microorganisms may be present. The main pathogens identified were Porphyromonas gingivalis, Prevotella intermedia, Actinobacillus actinomycetemcomitans (Aggregatibacter actinomycetemcomitans), Bacteroides forsythus (Tannerella forsythensis) , and Treponema. Numerous studies have indicated the composition of the microflora associated with periodontitis and peri-implantitis (bleeding and bone loss around an implant) are similar. Furthermore, when Shibli and colleagues compared the microflora around implants that manifested peri-implantitis and those that were healthy, it was noted that the same types of bacteria were present around diseased and healthy implants; but an increased quantity of bacteria was found at diseased sites.
With respect to the rate that bacteria from a tooth colonize an implant, Quirynen and colleagues reported that initial subgingival colonization of implants with bacteria associated with periodontitis can occur within 2 weeks in partially edentate patients. On the other hand, in completely edentate individuals, Danser and colleagues noted that the main reservoir of colonization for dental implants in edentulous patients was oral mucous membranes. After all teeth were removed due to periodontitis, they found that bacteria harbored by individuals with dental implants were species usually associated with a healthy periodontium or gingivitis. It was suggested that extraction of natural teeth resulted in elimination of two potential pathogens, A actinomycetemcomitans and P gingivalis . In contrast to these findings, however, others indicated that implants placed into edentate individuals experienced re-emergence of bacterial pathogens by 6 months with an almost identical spectrum of pathogens, including P gingivalis, T forsythensis, and other pathogenic bacteria that were present before the teeth were extracted.
In summary, the finding that bacteria associated with implant health and disease are similar is not surprising, because most microbes located in the oral cavity are considered indigenous organisms. Furthermore, it appears that teeth and other reservoirs of bacteria (mucous membranes, saliva, pharynx) in edentate patients have the potential to be a source of bacterial reinfection once implants are placed. This underscores the need to initiate periodontal therapy in patients with periodontitis before placing dental implants to reduce the level of potential pathogens, thereby inhibiting them from colonizing the implants and initiating peri-implantitis.
Etiologic agents of periodontitis and peri-implantitis
Bacteria are the main etiologic agents that induce periodontitis and peri-implantitis ; therefore, knowledge concerning the main pathogens is important for understanding the linkage between retention of implants and a patient’s history of periodontitis. Within the human oral cavity, hundreds of species of bacteria have been identified. In individuals with chronic periodontitis, it has been reported that the predominant bacterial species are gram-negative anaerobes; however, other microorganisms may be present. The main pathogens identified were Porphyromonas gingivalis, Prevotella intermedia, Actinobacillus actinomycetemcomitans (Aggregatibacter actinomycetemcomitans), Bacteroides forsythus (Tannerella forsythensis) , and Treponema. Numerous studies have indicated the composition of the microflora associated with periodontitis and peri-implantitis (bleeding and bone loss around an implant) are similar. Furthermore, when Shibli and colleagues compared the microflora around implants that manifested peri-implantitis and those that were healthy, it was noted that the same types of bacteria were present around diseased and healthy implants; but an increased quantity of bacteria was found at diseased sites.
With respect to the rate that bacteria from a tooth colonize an implant, Quirynen and colleagues reported that initial subgingival colonization of implants with bacteria associated with periodontitis can occur within 2 weeks in partially edentate patients. On the other hand, in completely edentate individuals, Danser and colleagues noted that the main reservoir of colonization for dental implants in edentulous patients was oral mucous membranes. After all teeth were removed due to periodontitis, they found that bacteria harbored by individuals with dental implants were species usually associated with a healthy periodontium or gingivitis. It was suggested that extraction of natural teeth resulted in elimination of two potential pathogens, A actinomycetemcomitans and P gingivalis . In contrast to these findings, however, others indicated that implants placed into edentate individuals experienced re-emergence of bacterial pathogens by 6 months with an almost identical spectrum of pathogens, including P gingivalis, T forsythensis, and other pathogenic bacteria that were present before the teeth were extracted.
In summary, the finding that bacteria associated with implant health and disease are similar is not surprising, because most microbes located in the oral cavity are considered indigenous organisms. Furthermore, it appears that teeth and other reservoirs of bacteria (mucous membranes, saliva, pharynx) in edentate patients have the potential to be a source of bacterial reinfection once implants are placed. This underscores the need to initiate periodontal therapy in patients with periodontitis before placing dental implants to reduce the level of potential pathogens, thereby inhibiting them from colonizing the implants and initiating peri-implantitis.
Survival of implants in patients with a history of periodontitis
Individuals who were fully or partially edentate were successfully rehabilitated using osseointegrated dental implants to support fixed prostheses. The question remains as to whether these individual are at greater risk of developing peri-implantitis than patients who have not previously had periodontal diseases, and if they are, how great are the risks and what can be done to enhance successful therapy with implants.
Partially Edentulous Patients
Karoussis and colleagues conducted a systematic review of the literature with respect to the success/survival rates of dental implants placed in patients with a history of periodontis who were partially dentate. Studies were assessed in two categories: short term (< 5 years) and long term (> 5 years) after osseointegrated implants were placed in periodontally compromised partially edentulous patients. Based upon 15 prospective investigations (seven short- and 8 long-term studies), the authors made the following observations. They found no statistically significant differences in the survival rates between the short- and long-term studies. However, when patients with a history of periodontitis were compared with individuals who were periodontally healthy, it was reported that patients with a history of periodontitis manifested significantly greater probing depths, more peri-implant marginal bone loss, and a higher incidence of peri-implantitis. It was concluded that implant survival rate was acceptable in individuals with a history of periodontitis who were in a maintenance program.
Fully Edentulous Patients
Several studies addressed the 15- to 20-year survival rates of implants placed in patients who were fully edentulous: For example, Astrand and colleagues found a 99.2% survival rate for implants; Adell and colleagues reported implant retention in the maxilla and mandible was 78% and 86%, respectively, and Jemt and Johansson reported that the implant survival rate was 90.9%. It can be surmised that the long-term survival rates with implants seem satisfactory in edentate patients. It should be recognized, however, that these clinical trials did not specify that the involved patients had a history of periodontitis, which may affect the long-term survival rate.
Patients with a History of Aggressive Periodontitis
To clarify the success rate of implants in patients with a history of aggressive periodontitis (ie, juvenile and rapidly progressive periodontitis), Al-Zahrani conducted a systematic review, which included nine articles, four of which were case reports. These publications demonstrated there was good short-term survival of implants placed in patients treated for aggressive periodontitis that subsequently were periodontally maintained. The data indicated, however, that bone loss occurred around implants in patients with a history of aggressive periodontitis more often than around implants in patients with history of chronic periodontitis or periodontally healthy individuals. In addition, the author made several comments that should be underscored to interpret these findings:
-
Periodontal diseases should be controlled before placement of implants.
-
Individuals with aggressive periodontitis may be susceptible to additional periods of disease progression. At present, however, no recommendations can be made to define a time period that should elapse before initiating implant therapy.
-
There are a limited number of studies addressing the survival rate in patients with aggressive periodontitis.
-
It is unknown what effect retention of questionable teeth in these patients will have on the success rate of implants in individuals who had aggressive periodontitis.
Implant Placement in Sites Augmented with Guided Bone Regeneration
Because patients with a history of periodontitis often require guided bone regeneration (GBR) procedures (bone graft with a barrier) to restore bone before implant placement, it was considered important to address survival of implants in grafted bone. A recent systematic review (11 studies included) by Hammerle and colleagues compared survival of implants in regenerated bone attained with GBR with survival of implants placed into native bone. The cumulative survival rates for implants in regenerated bone varied from 79.4% to 100% after 5 years of function. The authors concluded that there were no significant differences found in the controlled clinical trials with respect to survival rates between implants placed in regenerated bone compared with implants inserted in native bone. It should be recognized, however, that this review did not specifically look at patients with a history of periodontitis, which may affect the implant survival rate.
Implants in Sinus Augmented Bone
Several investigations specifically assessed the survival rate of implants placed into sinus grafts in patients who were periodontally compromised. Ellegaard and colleagues monitored 24 patients for 36 to 42 months and 68 patients for 10 years. Both studies concurred that implants may be inserted into a sinus augmented with bone in periodontally compromised patients with the same success as implants placed in individuals without a history of periodontitis.
Diagnostic parameters to assess dental implants
The tissues that surround teeth and implants respond in a similar manner to a bacterial challenge. Peri-implant diseases consist of two entities: peri-implant mucositis that is similar to gingivitis, and peri-implantitis, which is comparable to periodontitis. Mucositis denotes that there is inflammation of the tissue around an implant without any signs of bone loss. In contrast, peri-implantitis connotes mucosal inflammation and bone loss. There are a few parameters that can be used to diagnose peri-implant diseases.
Probing Depth
Deeper than usual probing depths around an implant may not indicate the presence of peri-implantitis, because an implant placed at various depths subgingivally can result in a deep sulcus. Contributing to this finding is the fact that connective tissue fibers adhere to, but are not attached to an implant as they are to teeth; therefore, they do not impede probe tip penetration. Nevertheless, increasing probing depth over time is associated with loss of bone around an implant. In health, the probe will penetrate to the apical extent of the epithelium, and the junctional epithelium heals within 5 days. In peri-implantitis lesions, the probe will penetrate into the connective tissue. Stable probing depths in the absence of recession reflect stability of the tissues adjacent to the implant. In summary, it is prudent to probe around dental implants to assess periodontal peri-implant health during periodic examinations.
Bleeding Upon Probing
Healthy implant sites manifest an absence of bleeding, whereas sites with mucositis or peri-implantitis demonstrate bleeding upon probing 67% and 91% of the time, respectively. Most importantly, it has been reported that an absence of bleeding is an indicator for a stable peri-implant condition with respect to future attachment loss.
Radiographs
Radiographs are a valuable aid in diagnosing loss of osseous support around an implant. Assessment of bone loss from the osseous crest to a fixed reference point (eg, osseous crest to implant–abutment connection) can be recorded; however, limitations of radiographs should be noted. For example, panoramic films have distortion of about 23%. Furthermore, in general, radiographs underestimate bone loss, because a substantial amount of the buccal or lingual plate of bone needs to be demineralized before it seen radiographically. Additionally, there is an inability to differentiate between defects in the buccal or lingual plate of bone. In contrast, computed tomography (CT) and cone beam volume imaging have provided accurate three-dimensional imaging of bone surrounding dental implants.
Biochemical and Other Markers of Disease
There are no biochemical markers from the peri-implant crevicular fluid or microbiological tests that are good predictors for future disease progression around dental implants. Mobility is not a useful clinical parameter to monitor implants, because its presence denotes a failed implant that needs to be removed. On the other hand, suppuration reflects an infection, but its presence may or may not denote the presence of ongoing bone loss.
Risk indicators for peri-implantitis
Several risk indicators for peri-implantitis identified in cross-sectional and retrospective studies were investigated. These indicators, however, are not necessarily true risk factors (delineate a cause and effect relationship), which can be identified only by prospective clinical trials.
History of Periodontis
Heitz-Mayfield assessed four systematic reviews that addressed the history of periodontitis as a risk factor for peri-implantitis. Despite variations in clinical trials with respect to their design and maintenance schedules, it was concluded that patients with a history of periodontitis are at greater risk for peri-implantitis than individuals who never have had periodontitis.
Diabetes
Only one investigation evaluated the relationship between peri-implantitis and diabetes. Ferreira and colleagues concluded that poor metabolic control in subjects with diabetes was associated with peri-implantitis (odds ratio was 1:9).
Genetics
Cytokine gene polymorphisms may alter the host response to a bacterial challenge and affect susceptibility to peri-implantitis. A recent systematic review by Huynh-Ba and colleagues found that there is not enough evidence to support an association between the interleukin (IL)-1 genotype status and peri-implantitis. Therefore, at present, genetic testing for the evaluation of the risk of peri-implantitis cannot be suggested as a standard of care.
Smoking
A systematic review (included six studies) by Strietzel and colleagues noted that there was a significant increase in marginal bone loss around implants in smokers compared with nonsmokers. It was concluded that smokers are at increased risk of biologic complications (eg, peri-implantitis and reduced implant survival rate) compared with nonsmokers.
Oral Hygiene
Two investigations indicated that poor oral hygiene was a risk indicator for peri-implantitis. Ferreira and colleagues noted that individuals with very poor oral hygiene had an increased odds ratio (14:3) of experiencing peri-implantitis compared with patients with good oral hygiene. Similarly, Linquist reported that after 10 years, smokers with poor oral hygiene (plaque accumulation was monitored) had three times greater marginal bone loss than nonsmokers.
Absence of Keratinized Tissue
The role of keratinized gingiva in maintaining dental implants is a controversial issue. Based on long-term implant success and survival studies, there appears to be little or no difference in the survival rate for implants surrounded by oral mucosa or keratinized tissue.
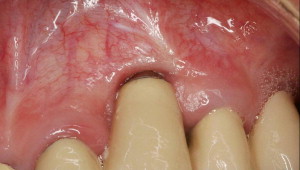
One paper, however, indicated that hydroxy apatite (HA)-coated implants had a higher survival rate when keratinized tissue was present. With respect to tissue inflammation, recession, and bone loss, there is conflicting information in the literature. When there was a dearth of keratinized gingiva, several investigators reported there was a statistically significant increased amount of inflammation, whereas others indicated the amount of inflammation was not increased when there was a lack of keratinized gingiva. Some researchers found that the absence of keratinized gingiva was associated with a statistically significant increased amount of recession ( Fig. 1 ), but this finding conflicted with the data of others. Similarly, several studies concluded that the absence of keratinized gingiva was or was not associated with a statistically significant additional bone loss. A possible explanation for these contradictions is that with good oral hygiene, peri-implant soft tissue health can be maintained irrespective of the amount of keratinized gingival tissue surrounding implant/restoration present; however, if there is less than good oral hygiene, it may be advantageous to have keratinized gingiva. In conclusion, despite a lack of data, some authors suggest that there may be situations when soft tissue augmentation at implant sites may need to be considered (eg, depending upon the site, dental history of the patient). These recommendations to date, however, have no scientific basis.
Implant Surfaces
It should be noted that there are three types of surface roughness (Sa) on implants (minimally rough, Sa = 0.5 μm also referred to as machined implants, smooth, turned), moderately rough, Sa equal to 1 to 2 μm (eg, Osseotite [3i Implant Innovations, FL, USA], SLA [Straumann Company, MA, USA], TiUnite [Nobel Biocare, CA, USA]) and rough, Sa greater than 2μm, (eg, plasma-sprayed and HA-coated implants) and that with increasing roughness, implant surfaces attract and retain more bacteria. There is limited and conflicting information, however, with respect to the impact of implant surface topography as a risk factor for peri-implantitis. In a dog model, investigators noted that the progression of peri-implantitis, if left untreated, is greater around implants with a moderately rough surface than those with a polished surface. In humans, Astrand and colleagues also found that rough-surfaced implants had a higher incidence of peri-implantitis than smooth (turned) surfaces, whereas, Wennstrom and colleagues reported similar bone level changes for turned and relatively rough surface implants. Albouy and colleagues compared the amount of induced disease progression (dog model) with respect to four different surfaces (turned, TioBlast [Astra Tech Inc, MA, USA], sandblasted acid-etched, and TiUnite). They reported that disease progression was most pronounced at implants with a TiUnite surface. In summary, at present, there is insufficient information in people to make a definitive determination as to whether the surface characteristic of the implant predisposes a patient to peri-implantitis.
Prevention, prevalence, and therapy for patients with peri-implantitis
Prevention of Peri-implantitis: Periodontal Supportive Therapy
It is appropriate that clinicians maintain a recall system for patients who receive implants in order to monitor them and provide supportive periodontal therapy (SPT). In this regard, Quirynen and colleagues reviewed 16 studies and concluded that periodontally compromised patients can be maintained successfully with moderately rough implants if they are provided SPT. With respect to the time interval between SPT visits, numerous investigations indicated that a 3-month interval is adequate for most periodontal patients ; but some patients need more or less frequent visits.
Prevalence of Peri-Implantitis
Zitzmann and Berglundh conducted a literature review to determine the prevalence of peri-implant mucositis and peri-implantitis. For studies to be included in their assessment, patients needed to have been monitored more than 5 years and included more than 50 subjects. Only two investigations met these criteria. The data were reported with regard to the percentage of implants and percentage of patients who manifested peri-implant diseases. The investigators noted that after 5 years that peri-implant mucositis (bleeding upon probing) was found in approximately 80% of the subjects and around 50% of the implants. In the two groups included in the systematic review, peri-implantitis (bleeding and bone loss) was detected as follows: group 1-28% of the subjects and 12% of the sites: group 2-greater than 56% of subjects and at 43% of implant sites (Branemark implants [Nobel Biocare, CA, USA]). Accordingly, it can be concluded that over time the prevalence of peri-implant diseases is greater than previously expected.
Treatment for Peri-Implant Diseases
Nonsurgical therapy
Renvert and colleagues selected 24 studies to assess nonsurgical therapy for mucositis and peri-implantitis. They reported that nonsurgical mechanical therapy could be used effectively to treat mucositis. Furthermore, antimicrobial mouth rinses improved the outcome of mechanical therapy. For peri-implantitis, however, nonsurgical therapy was not found to provide satisfactory outcomes, and adjunctive rinsing with chlorhexidine had limited value. Local or systemic drug delivery helped decrease bleeding upon probing and probing depths ; however, it could not resolve peri-implantitis. They also indicated that laser therapy has the potential to be efficacious, but there are not enough data at this time to judge its effectiveness as a nonsurgical treatment modality.
Surgical therapy
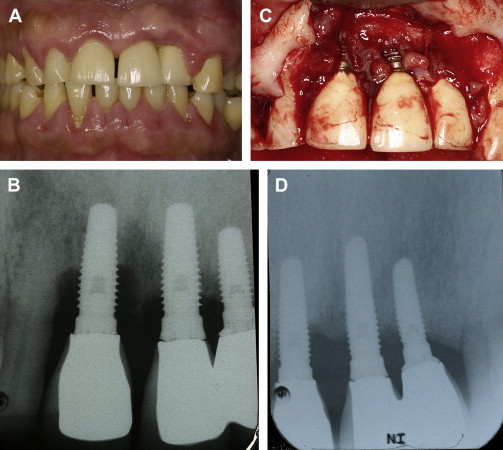
Claffey and colleagues evaluated information gathered from animal and human clinical trials concerning surgical therapy for peri-implantitis. Histologic data from animal studies validated that reosseointegration to contaminated surfaces was attainable, but not predictably. No single method of decontaminating the roots (eg, chemical agents, air abrasives and lasers) appeared to be distinctly better than other techniques. It was concluded that open debridement with surface decontamination can resolve peri-implantitis ( Fig. 2 ). With respect to people, one study indicated that therapy was successful in 58% of the patients. Nevertheless, at present there does not appear to be a best treatment of peri-implantitis. Furthermore, bone grafts with and without barriers have been used with varying degrees of success.