INTRODUCTION
Uses of Dental Cements
Classification of Dental Cements
ZINC OXIDE EUGENOL CEMENT
Composition
Setting Reaction of Zinc Oxide Eugenol Cement
Manipulation of Zinc Oxide Eugenol (ZOE) Cement
Biocompatibility of Zinc Oxide Eugenol Cement
Types of ZOE
Clinical Uses
ZINC PHOSPHATE CEMENT
Composition
Setting Reaction
Manipulation of Cement
Mechanical Properties
Biocompatibility
Clinical Uses
ZINC SILICOPHOSPHATE CEMENTS (ZSPC)
ZINC POLYCARBOXYLATE CEMENT
Composition
Manipulation of Zinc Polycarboxylate Cement
Setting Reaction
Bonding of Polyacrylate Cement to Tooth Structure
Mechanical Properties
Solubility
Biological Considerations
Uses of Zinc Polycarboxylate Cement
GLASS IONOMER CEMENT
Classification of Glass Ionomer Cements
Composition of Glass Ionomer Cement
Water Settable Glass Ionomer
Metal Reinforced Glass Ionomer Cement
Resin Modified Glass Ionomer
Manipulation of Glass Ionomer Cement
Manipulation of Glass ionomer cement – Capsule Form
SETTING REACTION
Setting Reaction of Autocure Glass Ionomer Cement
Setting Reaction of Resin Modified Glass Ionomers
Setting Time
INDICATIONS OF GLASS IONOMER CEMENT
CONTRAINDICATIONS OF GLASS IONOMER CEMENTS
PROPERTIES OF GLASS IONOMER CEMENT
Physical Properties
Biocompatibility
Water Sensitivity
Adhesion
Fluoride Release
Esthetics
Margin Adaptation and Leakage
Radiopacity
RESIN CEMENTS
Uses
Types of Resin Cements
Technique for Using Resin Cements
INTRODUCTION
Dental cements: Dental cements are the materials made from two components, powder and liquid when mixed together form a pastelike or flowable material which hardens to a solid structure.
Uses of Dental Cements
Dental cement can be used as:
• Temporary restoration
• Permanent restoration
• Temporary luting
• Permanent luting
• Root canal sealing
• Pulp protection
– bases
– liners/pulp capping agents
– varnishes
Different uses of cements are determined by:
• Composition
• Compressive strength
• Modulus of elasticity
• Film thickness
• Solubility
• Biocompatibility
Classification of Dental Cements
Based on Composition
i. Conventional cement
• Zinc phosphate cement
• Zinc oxide eugenol cement
• Polycarboxylate cement
• Glass ionomer cement
ii. Resin-base cement
• Resin cement
• Resin modified glass ionomer cement
Classification Based on Uses
1. Permanent luting cements:
• Zinc phosphate
0 Zinc silicophosphate
0 Zinc polycarboxylate cement
• Modified ZOE type-II
• Glass ionomer cement
2. Temporary luting cements:
• ZOE type I
3. Permanent restoratives:
• Silicates
• GIC type II
4. Temporary restorative materials:
• ZOE (reinforced) type III
• GIC type II
5. Bases:
A. Under amalgam:
• Zinc phosphate
• Zinc silicophosphate
• Zinc polycarboxylate
• Reinforced ZOE (type III)
• GIC (type III)
• Ca(OH)2
B. Bases under composites:
• Zinc polycarboxylate
• GIC type III
• Ca(OH)2
C. Bases under gold:
• Zinc phosphate
• Zinc polycarboxylate
• GIC (type III)
6. Pulp capping agents:
i. Indirect pulp capping agents
• Ca(OH)2
• ZOE
ii. Direct pulp capping agents
• Ca(OH)2
7. Cavity liners
i. Under amalgam
• Ca(OH)2
ii. Under composite
• Ca(OH)2
• GIC type III
• Varnishes
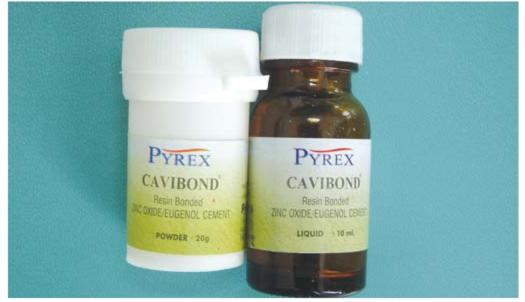
ZINC OXIDE EUGENOL CEMENT (FIG. 8.1)
Zinc oxide eugenol cement is one of the oldest used cement. Since, it has soothing action on pulpal tissues and eugenol has topical anesthetic properties. Hence, it is termed an obtundent material. Though other cements are also used for temporization, but zinc oxide eugenol cement is used most commonly because zinc oxide eugenol cements are much less irritant to the pulp, less soluble in oral fluids and produce better marginal seal than zinc phosphate. A thick mix of zinc oxide eugenol cement is used for small tooth preparations, but before placing the cement, the prepared surface must be isolated and cleansed. Zinc oxide eugenol is not used as base material especially when unfilled and filled resins are used as restorative materials because eugenol interferes with polymerization process of resins. In these cases calcium hydroxide is used as base material under resin restoration.
Fig. 8.1: Zinc oxide eugenol cement
Composition
a. Powder
• Powder Zinc oxide (ZnO)-69.0%-Reactive ingradient
• White rosin-29.3%-Reduces brittleness
• Zinc stearate-1.0%-Catalyst
• Zinc acetate (acts as accelerator) -0.7%Accelerator
b. Liquid
• Eugenol-85.0%-Reactor
• Olive oil-15.0%-Plasticizer.
Setting Reaction of Zinc Oxide Eugenol Cement
On mixing powder and liquid, the zinc oxide hydrolysis and subsequent reaction takes place between zinc hydroxide and eugenol to form a chelate, zinc eugenolate. Within this matrix unreacted zinc oxide powder particles are embedded.
• First reaction
ZnO + H2O – Zn (OH)2
• Second reaction
Zn(OH)2 + 2HE – ZnE2 + 21120
• Water is needed for the reaction and it is also a byproduct of the reaction so reaction progresses more rapidly in humid conditions.
• Because zinc eugenolate rapidly hydrolyzes to form free eugenol and zinc hydroxide, it is one of the most soluble cements. To increase the strength of the set material, changes in composition can be made to the powder and liquid. For example, ortho-ethoxybenzoic acid can be added to the liquid or alumina or polymethyl methacrylate powder can be added to the powder. These modified zinc oxide-eugenol cements (Fig. 8.2) have the following compositions:
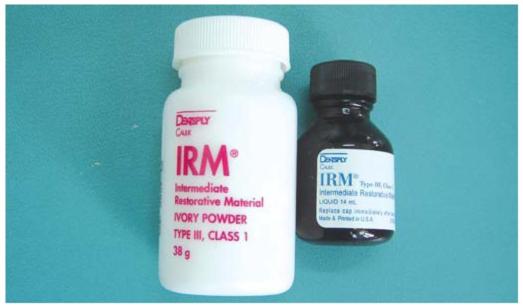
Fig. 8.2: Intermediate restorative material; modified zinc oxide eugenol cement
Alumina and Ortho-ethoxybenzoic Acid Reinforced Composition
a. Powder
b. Liquid
EBA (Ortho-ethoxybenzoic acid) cement: In this cement, EBA chelates with zinc forming zinc benzoate. Addition of fused quartz, alumina and dicalcium phosphate has also shown to improve mechanical properties of cement.
Effect of EBA on Eugenol Cement
1. Increase in compressive and tensile strength.
2. More powder can be incorporated to achieve standard consistency.
3. Decrease in setting time (if concentration is < 70%).
4. EBA does not show adverse effects on pulp.
Polymer Reinforced Zinc Oxide
a. Powder
b. Liquid
Polymer reinforced zinc oxide eugenol cement: In this mixture, resin helps in improving strength, smoothness of the mix and decreasing flow, solubility and brittleness of the cement.
Manipulation of Zinc Oxide Eugenol (ZOE) Cement
• ZOE are available as: (i) powder and liquid system, (ii) paste-paste system.
Manipulation of Powder and Liquid System
Powder is measured and dispensed with a scoop where as liquid is dispensed as drops on glass slab.
• Powder is dispensed at one end of glass slab, using cement spatula. The powder is divided in main bulk increment, followed by smaller increments (Figs 8.3 and 8.4). While dispensing liquid, bottle should be held 90° to the mixing pad. It lets the fluid fall under its own weight.
Fig. 8.3: Dispensing of zinc oxide eugenol powder and liquid
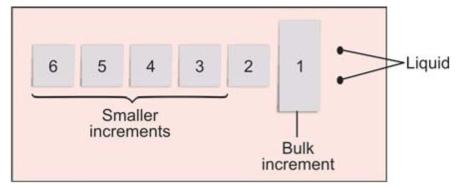
Fig. 8.4: Diagrammatic representation of increments of zinc oxide powder
• Start the mixing by incorporating half of the powder into the liquid with a heavy folding motion and pressure.
• When powder particles are wet with the liquid, add the remaining powder to the mix and continue to use a heavy folding motion to attain a putty consistency.
• For base, when mixing is done, bring the mix together and role it. One should be able to pick up the mix without deformation (Fig. 8.5).

Fig. 8.5: Right consistency of ZOE mix
• Pick-up a piece of mixed cement and place it into the preparation using a condenser.
• If the mix sticks to the condenser, powder the condenser head with cement powder to prevent the instrument from sticking during condensation.
• Use the condenser head and merge the restoration to the margin of the preparation.
• For smoothening, clean up and hardening of the restoration, use a wet cotton pellet.
• For luting consistency, “1 inch” string should be formed when flat surface of spatula is pulled from the mixed cement.
Paste-paste System
In this two pastes are dispensed in equal lengths on paper pad. Two pastes have different colors, mixing is done till a homogeneous color is obtained.
Working Time and Setting Time
These are usually not specified. In general, higher is the P:L, faster the materials sets. Cooling of glass slab slows down the setting reaction (unless the temperature is below the dew point).
Setting time of this cement is long but since water accelerates the setting reaction, it sets faster in mouth than outside.
Biocompatibility of Zinc Oxide Eugenol Cement
ZOE is the best known obtundent. pH of cement is 7. This makes it least irritating cement. Because of this it is considered most palliative agent to the pulp.
Types of ZOE
Type I – Temporary luting
Type II – Long-term luting
Type III – Temporary restoration
Type IV – Intermediate restoration
Type I
Main features:
• Strength of the cement is low so it can be easily removed.
• It is used for short-term restorations.
• Free eugenol interferes with the setting of resin bonded composites so carboxylic acids can be used to replace eugenol making it non eugenol cement.
Type 11
Main features:
• This cement has improved strength and abrasion resistance.
• In this cement the part of eugenol liquid substituted by ortho-ethoxybenzoic acid (EBA) and alumina is added to powder.
or
• Powder is made up of 20 wt percent to 40 wt percent of fine polymer particles and zinc oxide particles that have been surface treated with carboxylic acid, in this the liquid remains eugenol.
Type 111
It is used for temporary restorations which last for a few days to few weeks.
Type IV
It lasts for atleast up to 1 year. In this more powder has to be added for achieving better strength.
Clinical Uses
• Base
• Temporary cementation
• Temporary restoration
• Root canal sealer
• Liner
• Pulp capping agent
• Permanent cementation
Advantages
• Soothing effect on the pulp
0 Good short-term sealing.
Disadvantages
• Highly soluble
• Low strength
• Long setting time
• Low compressive strength.
ZINC PHOSPHATE CEMENT (FIG. 8.6)

Fig. 8.6: Zinc phosphate cement
One of the oldest and most widely used cements, zinc phosphate cement is the standard against which new cements are compared. It was first introduced in 1878 and still used today because of excellent clinical track record.
Two Types
Type I.• Used for cementation. Specification requires the film thickness of less than 25 microns.
Type II: Used as a base and for luting. Specification requires a film thickness between 25 and 40 microns.
Composition
Powder: The primary ingredients of zinc phosphate cement powder are zinc oxide and magnesium oxide.
• ZnO-90.2%.
• MgO-8.2%-condenses the ZnO during the sintering process
• Si02-1.4%-acts as an insert filler
• Bi203-0.’%-imparts smoothness to the mixed cement
• Miscellaneous-(BaO, Ba2SO4, CaO) – 0.1%.
All the ingredients are sintered at temperatures between 1000°C and 1400°C into a cake that is subsequently ground into fine powder.
Liquid: The liquid is phosphoric acid and water in the ratio of two parts acid to one part water. It may also contain aluminium phosphate and zinc phosphate. The water content of the liquid is critical and should be controlled to provide a adequate setting time. When the liquid is exposed in open bottle, it absorbs moisture from the air in case of high humidity but in low humidity times it will lose moisture. In case of very old liquids, the last 25% portion remaining in the bottle should be discarded because it is usually discolored or contaminated.
Both aluminium and zinc act as buffers to reduce the reactivity of the powder and liquid.
Setting Reaction
Phosphoric acid attacks surface of the particles and releases Zn ions into the liquid. Aluminium which already forms a complex with the phosphoric acid reacts with zinc and yields a zincaluminophosphate gel
The set cement consists of a zinc phosphate matrix in which unreacted zinc oxide powder particles are embedded. Crystals of tertiary zinc phosphate/ hopeite, are found on the surface of the cement (Fig. 8.7).
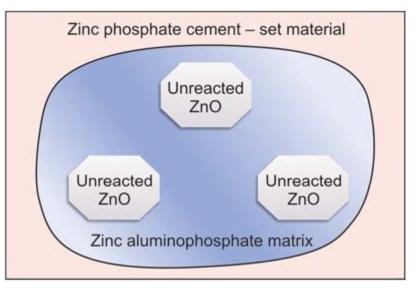
Fig. 8.7: Structure of set zinc phosphate cement
Manipulation of Cement
Manipulation of Zinc Phosphate Cement
• Working time – 5 minutes
0 Setting time — 2.5 to 8 minutes
• Powder is measured and dispensed with scoop a liquid is dispensed as drops. Cement mixing should be done on cool glass slab with a narrow bladed stainless steel spatula. Lower the temperature of the slab during mixing, the longer will be the working time. This is advantageous because it allows incorporation of more powder into the liquid which results in greater compressive strength and lower solubility of the final cement.
• Some clinicians prefer to mix the cement using the “frozen slab” technique which greatly extends the working time and allows incorporation of more powder into the liquid. But this method has disadvantage of incorporating water into the mix.
• Since setting reaction is an exothermic type the heat liberated while setting further accelerates the setting rate. So it is very important to dissipate this heat which can be done by
a. Using chilled glass slab.
b. Using smaller increment for initial mixing of cement.
c. Mixing an large area of glass slab
• Powder is divided into 5-8 increments (Figs 8.8A and B) in which initial two increments are smaller, third and fourth increments are bigger one and after that increments are again smaller in size.

Fig. 8.8A: Diagrammatic representation of increments of zinc phosphate powder
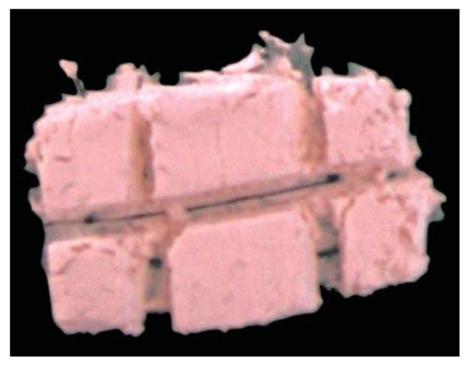
Fig. 8.8B: Photograph showing increments of zinc phosphate powder
• Initial increments are smaller in size so as:
– To achieve the slow neutralization of the liquid.
– To control the reaction.
• Middle increments are larger in size so as to further saturate the liquid to form zinc phosphate. Because of presence of less amount of unreacted acid, this step is not affected by heat released from the reaction.
• In the end the smaller increments of powder are added so as to achieve optimum consistency
• After dividing powder, dispense liquid on the glass slab. While dispensing, the liquid bottle should be held vertical and close to the powder (Fig. 8.9). Repeated opening of the liquid bottle or early dispensing of the liquid prior to mixing should be avoided because evaporation of liquid can result in changes in water/acid ratio which can further result in decrease in pH and an increase in viscosity of the mixed cement.

Fig. 8.9: While dispensing liquid, the bottle should be held vertical and close to the powder
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


