Introduction
The aim of this study was to evaluate the antimicrobial efficacy of 2 commercially available mouth rinses on a monospecies-biofilm model on orthodontic brackets in vitro.
Methods
The antimicrobial effects of the 2 mouth rinses, Listerine (tartar control; IDS Manufacturing, Bangkok, Thailand) and Corsodyl (SmithKline Beecham, Maidenhead, United Kingdom), on the planktonic Streptococcus mutans were tested by maximum inhibitory dilution assay. The cell viability of S mutans biofilm on Damon3 MX brackets (Ormco, Glendora, Calif) after exposure to the 2 mouth rinses was quantified by 2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide (XTT) reduction assay. Visualization of the biofilm samples was performed by fluorescence microscopy and confocal laser scanning microscopy.
Results
The maximum inhibitory dilution assays of S mutans were 1:5 for Listerine and 1:320 for Corsodyl. The optical density values, which were measured by XTT reduction assay from S mutans biofilms after 1 minute of exposure to the different test agents, demonstrated that the cell viability of S mutans biofilms exposed to Listerine was less than that for Corsodyl, which was less than that for brain-heart infusion ( P <0.001). Listerine caused more dead cells on the surface of the brackets than did Corsodyl when examined with the 2 microscope systems.
Conclusions
Both mouth rinses showed marked antimicrobial effects on the monospecies biofilm in vitro. Listerine showed a stronger bactericidal effect but had less bacterial inhibitory effect than did Corsodyl.
Bacterial plaque plays an essential role in the etiology of periodontal diseases and dental caries. Previous research has shown the placement of orthodontic brackets creates new locations for plaque retention, thereby increasing plaque adhesion and subsequent inflammatory responses. Therefore, aside from daily mechanical hygiene practices, chemical antimicrobial agents have been proposed to improve the efficiency of plaque control in orthodontic patients. The main advantage of mouthwashes is their ability to deliver antimicrobial benefits to all accessible surfaces in the mouth, while remaining active for an extended period of time.
Chlorhexidine, regarded as an effective antibacterial agent for chemical plaque control, has been routinely recommended to orthodontic patients. Listerine (tartar control; IDS Manufacturing, Bangkok, Thailand), a mouthwash containing essential oils, has also been reported to prevent and reduce supragingival plaque and gingivitis in orthodontic patients. However, the relative antimicrobial effectiveness of the various mouth rinses remains controversial. One clinical trial demonstrated that an essential-oil mouth rinse and 0.12% chlorhexidine have similar antiplaque activities, whereas other studies found that 0.12% chlorhexidine digluconate was superior to Listerine in maintaining gingival health. Furthermore, in a 1-year follow-up study, Listerine was reported to have an uncertain effect on plaque control. These contradictory results indicate that many factors can affect the outcomes of clinical trials.
Previous studies used several bacterial biofilm models to test the antimicrobial efficacy of mouth rinses. However, the antimicrobial effect of mouth rinses on Streptococcus mutans , which is the major pathogen for demineralization on the enamel surrounding orthodontic brackets, has not been fully investigated. Furthermore, the morphology and architecture of orthodontic brackets provide new areas of retention and prevent complete plaque removal, thus facilitating dental plaque accumulation and maturation. Therefore, determining the antimicrobial efficacy of mouth rinses on a biofilm model of orthodontic brackets in vitro might help us understand the antimicrobial mechanism in clinical situations. The aim of this study was to evaluate the antimicrobial efficacy of Listerine and Corsodyl (SmithKline Beecham, Maidenhead, United Kingdom) on an S mutans biofilm model on orthodontic brackets in vitro.
Material and methods
S mutans (strain ATCC 35668) was obtained from the archival collection of the Department of Oral Biosciences, Faculty of Dentistry, at the University of Hong Kong. Frozen isolates were thawed, and the identity reconfirmed by using the standard laboratory method. Bacterial species were inoculated on horse blood agar plates and incubated in an anaerobic chamber (5% carbon dioxide, 10% hydrogen, 85% of nitrogen) at 37°C for 2 days, and then microorganism cultures were harvested and suspended in brain-heart infusion at a concentration of 10 7 to 10 8 colony-forming units per milliliter (4.0 McFarland standard at 660 nm) for this experiment.
The maximum inhibitory dilution values of the 2 commercially available mouth rinses, Listerine and Corsodyl, were determined by using a broth microdilution assay with a modified protocol. Briefly, the mouth rinses were serial 2-fold diluted in brain-heart infusion to achieve final dilutions ranging from 1:2.5 to 1:1280, respectively, in a 96-well plate. The S mutans suspension was inoculated with serial dilutions of antimicrobial agents and adjusted to yield a cell concentration of 10 5 to 10 6 colony-forming units per milliliter (0.5 McFarland standard at 660 nm), each containing 200 mL of inocula, and a phosphate-buffered solution served as the negative control. The microplates were prepared in triplicate and incubated at 37°C, and readings were taken considering the maximum inhibitory dilution as the greatest dilution of the mouthwash capable of inhibiting visible growth (by the naked eyes) of the evaluated strain after overnight incubation growth.
Twelve mandibular incisor brackets (Damon3 MX; Ormco, Glendora, Calif) were chosen for the test. S mutans biofilm was developed on the brackets according to the previous protocol of biofilm formation on microtiter plates with a slight modification. Briefly, the sterilized brackets were placed on red utility wax (this step prevented the formation of S mutans biofilm on the bracket base), transferred to the 24-well plate, and incubated with 1000 μL of microorganism suspension brain-heart infusion with 1% sucrose at a concentration of 10 5 to 10 6 colony-forming units per milliliter (0.5 McFarland standard at 660 nm). Then the plates were incubated anaerobically at 37°C as a static culture for 24 hours.
After 24 hours of incubation, the 12 brackets were divided into 3 equal groups (2 experimental groups, 1 negative control group), placed in a 96-well plate, and then washed twice with 200 μL of brain-heart infusion to flush away the nonadhered bacteria. By washing the biofilm in this way, the disturbance effect of an air-liquid interface was avoided, since this might increase errors in cell enumeration. After that, 200 μL of the mouth rinses were separately added to the corresponding test groups and then discarded after 1 minute of static exposure; the brain-heart infusion served as a negative control with the same procedure above. Thereafter, each bracket was washed with 200 μL of brain-heart infusion twice, and subsequently 200 μL of 2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide (XTT) solution (containing 158 μL of phosphate-buffered solution and 40 μL of XTT, 2 μL diluted menadione) was added. The 96-well plate was incubated statically for 3 hours at 37°C in a dark cabinet. Then each solution was transferred to a new 96-well plate, and the absorbance was read in a spectrometer at 490 nm. The above procedures (including biofilm forming and XTT reduction assay) were performed on 3 separate occasions.
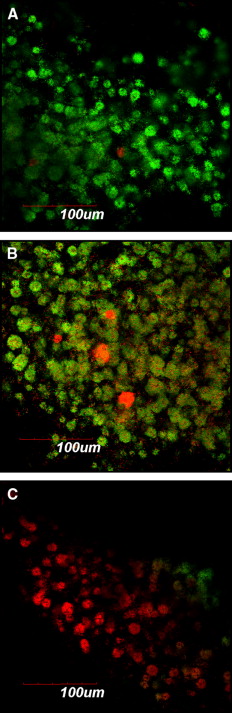
For fluorescence microscopy and confocal laser scanning microscopy imaging, S mutans biofilms were developed on brackets and exposed to different agents by using the same protocol as above. Thereafter, the biofilms on the brackets were gently washed twice with brain-heart infusion and stained with the molecular probes’ Live/Dead BacLight viability kit comprising SYTO-9 and propidium iodide (Invitrogen, Eugene, Ore). STYO-9 is a green fluorescent nucleic acid stain that generally labels live and dead microorganisms. Propidium iodide, in contrast, is a red fluorescent nucleic acid stain that penetrates only the cells with damaged membranes, thus visualizing only the dead microbes. The biofilms were incubated with SYTO-9 and propidium iodide for 20 minutes in a dark cabinet before the microscopic examinations. Subsequently, the structure and the bacterial vitality of the biofilms on the brackets were visualized by fluorescence microscopy and confocal laser scanning microscopy (FluoView FV 1000; Olympus, Tokyo, Japan), respectively.
Statistical analysis
Statistical analysis was performed with SPSS software (version 17.0; SPSS, Chicago, Ill). The mean values of the viability of the S mutans biofilm after exposure to the different agents were analyzed by 2-way repeated measures analysis of variance (ANOVA). Statistical significance was set at P <0.05.
Results
Broth microdilution assay demonstrated that both mouth rinses were effective against S mutans in the planktonic state. The maximum inhibitory dilution values were 1:5 for Listerine and 1:320 for Corsodyl. Listerine showed less inhibitory effect on planktonic S mutans than did Corsodyl.
The optical density values, which were measured by XTT reduction assay from S mutans biofilms after 1 minute of exposure to the different test agents, demonstrated that the cell viability of S mutans biofilms exposed to Listerine was less than that of Corsodyl, which was less than that for brain-heart infusion ( P <0.001) ( Table ). Subsequently, Listerine showed a stronger bactericidal effect on bacteria biofilm than did Corsodyl.
| Optical density | Listerine | Corsodyl | Brain-heart infusion |
|---|---|---|---|
| Mean ± SD (n = 4) | Mean ± SD (n = 4) | Mean ± SD (n = 4) | |
| Measurement 1 | 0.23 ± 0.02 | 1.48 ± 0.07 | 2.29 ± 0.04 |
| Measurement 2 | 0.45 ± 0.06 | 1.41 ± 0.21 | 2.12 ± 0.21 |
| Measurement 3 | 0.39 ± 0.14 | 1.71 ± 0.19 | 1.97 ± 0.16 |
The applied fluorescent vitality staining showed changes in the proportions of living vs dead bacteria upon treatment with the 2 mouth rinses. The images under the 2 microscope systems showed that both mouth rinses obviously increased the amounts of dead bacteria on the surfaces of the orthodontic brackets compared with the brain-heart infusion solution. Listerine killed more bacteria compared with Corsodyl ( Figs 1 and 2 ).