The management of a tooth with incomplete root maturation and a necrotic pulp is an endodontic and a restorative challenge. Apexification procedures alone leave the tooth in a weakened state and at risk for reinfection. Regenerative endodontic procedures potentially offer advantages, including the possibility of hard tissue deposition and continued root development. Case studies have reported regeneration of human pulplike tissues in vivo, but there is no protocol that reliably regenerates pulplike tissue. This article summarizes historical, current, and future regenerative treatment approaches.
- •
Case reports have indicated that biologically based endodontic therapies can result in the elimination of apical periodontitis. There is no question that regenerative endodontics procedures can be successful. The present issue is how to develop safe, effective and consistent methods for regenerating a functional pulp-dentin complex in patients.
- •
There are obvious benefits of regenerative endodontic procedures for the immature root; however, mature teeth may also benefit from the regeneration of a vital pulp-dentin complex. If the treatment plan of a tooth with extensive caries could be altered from certain extraction to being properly restored as a result of the regeneration of dentinal tooth structure, endodontists would have engineered a more desirable outcome.
- •
The reestablishment of vitality and a functional immune response may allow the body to better fight the presence of any remaining bacteria within the canal system. Thus, future efforts must focus on a more biologic and scientific approach to these endodontic procedures.
- •
Randomized prospective multicenter trials offer the potential to develop evidenced-based methodologies for these pioneering regenerative endodontic procedures.
Introduction
Regenerative endodontic procedures (REPs) can be defined as biologically based procedures designed to replace damaged structures including dentin and cells of the pulp-dentin complex. Case studies have presented clinically successful regenerative endodontic procedures in vivo ( Table 1 ), but the present information remains inadequate to define a single regenerative protocol. Despite published outcomes, clinicians continue to question the predictability of the procedure. Anecdotal evidence suggests moderate success rates and a need for more reliable protocols.
| Age (y) | Tooth No. Treated | Periapical Diagnosis (at Time of Presentation to Endodontic Clinic) | Irrigant(s) | Depth/Technique of Irrigation | Antimicrobial | Antimicrobial Placement Method | Duration of Antimicrobial | Tissue in Canal? | Evoke Blood Clot-Scaffold? | Pulp Space Barrier/Restoration | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Iwaya et al, 2001 | 13 | 29 | Chronic apical abscess | 5% NaOCl; 3% hydrogen peroxide (weekly for 5 wk) | Coronal portion of root | Metronidazole; ciprofloxacin (weekly for 5 wk) | No indication | 4 wk | Yes | Smooth broach inserted to vital tissue; no report of bleeding | Ca(OH) 2 ; glass ionomer; composite resin |
| Banchs and Trope, 2004 | 11 | 29 | Chronic apical abscess | 5.25% NaOCl; Peridex | Within 1 mm of apex | Metronidazole; ciprofloxacin; minocycline | Lentulo | 26 d | Yes | Endodontic explorer used to create bleeding 3 mm below CEJ | MTA; composite resin |
| Chueh and Huang, 2006 | 10 | 20 | Chronic apical abscess | 2.5% NaOCl | Pulp chamber | Ca(OH) 2 | No indication | 3 mo | Hard tissue barrier | None | Ca(OH) 2 ; Cavit; amalgam |
| 10 | 29 | Acute apical abscess | 2.5% NaOCl | Pulp chamber | Ca(OH) 2 | No indication | 8 wk | Hard tissue barrier | Hemorrhage observed | Ca(OH) 2 ; Cavit; Ketac Silver | |
| 10 | 20 | Chronic apical abscess | 2.5% NaOCl | Canal irrigated | Formocresol (before referral) Ca(OH) 2 | No indication | 1 mo | Hard tissue barrier | None | Ca(OH) 2 ; Cavit; glass ionomer; removed at 18.5 mo and amalgam placed | |
| 9 | 29 | Acute apical abscess | 2.5% NaOCl | Canal irrigated | Ca(OH) 2 | No indication | 5 wk | Hard tissue barrier | None | Ca(OH) 2 ; IRM; removed at 3y and amalgam placed | |
| Petrino 2007 | 8 | 8 | Chronic apical abscess | 5.25% NaOCl; Peridex | Within 1 mm of apex | Metronidazole; ciprofloxacin; minocycline | No indication | No indication | Yes; vital tissue felt | Endodontic explorer used to create bleeding 3 mm below CEJ | MTA; composite resin |
| Thibodeau and Trope, 2007 and 2009 | 9 | 8 | Acute apical abscess | 1.25% NaOCl | Canal irrigated | Metronidazole; ciprofloxacin; cefaclor | Lentulo and tamped down with sterile paper points | 11 wk | No | Endodontic file to create bleeding | wMTA; composite resin |
| Jung et al, 2008 | 10 | 29 | Chronic apical abscess | 5.25% NaOCl | Canal irrigated | (1) Metronidazole; ciprofloxacin; minocycline. (2) Erythromycin and Ca(OH) 2 . (3) Ca(OH) 2 | Lentulo | 88 d | Hard tissue barrier | None | Gutta-percha; composite resin |
| 28 | Chronic apical abscess | 5.25% NaOCl | Canal irrigated | Metronidazole; ciprofloxacin; minocycline | Lentulo | 67 d | Hard tissue barrier | None | Gutta-percha; composite resin | ||
| 10 | 29 | Chronic apical abscess | 5.25% NaOCl | Canal irrigated | Metronidazole; ciprofloxacin; minocycline | Lentulo | 11 d | Yes | None | MTA; composite resin | |
| 10 | 20 | Asymptomatic apical periodontitis (left open) | 5.25% NaOCl | Canal irrigated | (1) Metronidazole; ciprofloxacin; minocycline (2) Ca(OH) 2 | Lentulo | (1) 30 d (2) 40 d | Yes | Hemorrhage observed after 30 d | MTA; composite resin | |
| 13 | 20 | Symptomatic apical periodontitis | 5.25% NaOCl | Canal irrigated | Metronidazole; ciprofloxacin; minocycline | Lentulo | 2 wk | Yes | None | MTA; IRM | |
| 10 | 20 | Asymptomatic apical periodontitis (left open) | 2.5% NaOCl | Canal irrigated | Metronidazole; ciprofloxacin; minocycline | No indication | 1 wk | No | Endodontic file to create bleeding | MTA; composite resin | |
| 9 | 20 | Chronic apical abscess | 2.5% NaOCl | Canal irrigated | Metronidazole; ciprofloxacin; minocycline | Lentulo | 1 wk | Yes | Endodontic file to create bleeding | MTA; composite resin | |
| 14 | 29 | Chronic apical abscess | 2.5% NaOCl | Canal irrigated | Ca(OH) 2 (aqueous) | No indication | 1 wk | No | Endodontic file to create bleeding | MTA; composite resin | |
| 10 | 29 | Asymptomatic apical periodontitis (left open) | 2.5% NaOCl | Canal irrigated | Metronidazole; ciprofloxacin; minocycline | No indication | 3 wk | No | Endodontic file to create bleeding | Collatape; MTA; composite resin | |
| Cotti et al, 2008 | 9 | 8 | Chronic apical abscess | 5% NaOCl; 3% hydrogen peroxide | Coronal portion of root | Ca(OH) 2 powder | Plugger | 2 wk | Yes | Endodontic file to create bleeding | MTA; glass ionomer; composite resin |
| Shah et al, 2008 | 9–18 | 14 teeth | Chronic apical abscess; asymptomatic apical periodontitis | 2.5% NaOCl; 3% hydrogen peroxide | Canal irrigated | Formocresol | Cotton pellet | Subsequent appointment | No | Needle to create bleeding | Glass ionomer |
| Chueh et al, 2009 | 6–14 | 23 teeth | Chronic apical abscess; asymptomatic apical periodontitis; acute apical abscess | 2.5% NaOCl | Canal irrigated | Ca(OH) 2 | Loosely placed | 1–25 mo | Hard tissue barrier | No instrumentation OR Endodontic instrument to create bleeding | (1) Gutta-percha/composite or Amal. (2) MTA/gutta-percha/composite or amalgam. (3) Amalgam |
| Ding et al, 2009 | 8–11 | 3 (available for analysis) | Chronic apical abscess; acute apical abscess | 5.25% NaOCl | Gently flushed | Metronidazole; ciprofloxacin; minocycline | No indication | 1 wk | No indication | Endodontic file to create bleeding | MTA; composite |
| Shin et al, 2009 | 12 | 29 | Chronic apical abscess | 6% NaOCl/Saline/2% chlorhexidine | Coronal portion of root | None (1-step procedure) | N/A | N/A | Yes | Endodontic file to create bleeding | wMTA/composite |
| Reynolds et al, 2009 | 11 | 2 teeth | Chronic apical abscess | 6% NaOCl/Saline/2% chlorhexidine | 2 mm from apex | Metronidazole; ciprofloxacin; minocycline (composite bonding/no staining) | Needle 2 mm short of apex | 4 wk | No indication | Endodontic file to create bleeding | gMTA/composite |
| Kim et al, 2010 | 7 | 8 | Symptomatic apical periodontitis | 3% NaOCl | Canal irrigated | Metronidazole; ciprofloxacin; minocycline (staining/bleached) | Lentulo | 6 wk | No indication | Paper points to create bleeding | MTA/glass ionomer/composite |
| Petrino et al, 2010 | 6–13 | 5 teeth | Chronic apical abscess; asymptomatic apical periodontitis | 5.25% NaOCl/saline/Peridex | Canal irrigated | Metronidazole; ciprofloxacin; minocycline | No indication | 3 wk | No | Endodontic file to create bleeding and CollaPlug (2 teeth) | MTA/composite |
| Thomson and Kahler, 2010 | 12 | 20 | Chronic apical abscess | 1% NaOCl (with ultrasonic activation) | 2 mm from apex | Metronidazole; ciprofloxacin; minocycline | Lentulo | 6 wk | No indication | Endodontic explorer used to create bleeding 3 mm below CEJ | wMTA/glass ionomer/composite |
| Nosrat et al, 2011 | 9 | 30 | Symptomatic apical periodontitis | 5.25% NaOCl | Canal irrigated | Metronidazole; ciprofloxacin; minocycline | Placed with K file | 3 wk | Necrotic tissue | Endodontic file to create bleeding | CEM cement (calcium-enriched mixture)/glass ionomer/amalgam |
| 8 | 30 | Chronic apical abscess | 5.25% NaOCl | Canal irrigated | Metronidazole; ciprofloxacin; minocycline | Placed with K file | 3 wk | No indication | Endodontic file to create bleeding | CEM cement (calcium-enriched mixture)/glass ionomer/SSC | |
| Lenzi and Trope, 2012 | 8 | 8 | Inconclusive | 2.5% NaOCl | Canal irrigated | Metronidazole; ciprofloxacin; minocycline | Lentulo | 35 d | No indication | Endodontic file to create bleeding | MTA Angelus/composite |
| 9 | Asymptomatic apical periodontitis | 2.5% NaOCl | Canal irrigated | Metronidazole; ciprofloxacin; minocycline | Lentulo | 35 d | No indication | Endodontic file to create bleeding | MTA Angelus/composite | ||
| Cehreli et al, 2011 | 8–11 | 6 teeth | Asymptomatic apical periodontitis | 2.5% NaOCl | 1–2 mm below orifice | Ca(OH) 2 | Loosely placed | 3 wk | No indication | Endodontic file to create bleeding | MTA; glass ionomer; composite or amalgam |
| Torabinejad and Turman, 2011 | 11 | 4 | Symptomatic apical periodontitis | 5.25% NaOCl | Canal irrigated | Metronidazole; ciprofloxacin; minocycline | Amalgam carrier and endodontic pluggers | 22 d | No indication | No creation of bleeding; PRP placed | MTA/Cavit/amalgam |
| Iwaya et al, 2011 | 7 | 24 | Acute apical abscess | 5% NaOCl; 3% hydrogen peroxide | Coronal portion of root (×5) | Ca(OH) 2 | 6 wk | Hard tissue barrier | None | Gutta-percha/composite resin |
Tissue Engineering
The ability to predictably regenerate natural tissues and create new tissues is the objective of the emerging field of tissue engineering. Nakashima described 3 essential components of tissue engineering: stem/progenitor cells, morphogenetic signals and three-dimensional (3D) scaffolds. Stem cells, morphogens/growth factors, and biomimetic scaffolds all play essential roles in the restoration of previously damaged structures. By addressing the 3 elements of tissue engineering in future clinical guidelines, it is hoped to provide a microenvironment that is conducive to regeneration of pulplike tissues in patients.
Commitment to regenerative endodontics
Regenerative endodontics is a high priority for the specialty of endodontics. The number of publications in peer-reviewed journals related to regenerative endodontics has increased greatly in recent years ( Fig. 1 ). Organized endodontics is committed to identifying evidence-based methodologies for these procedures. The next logical phase of regenerative endodontic research will be prospective randomized clinical trials.
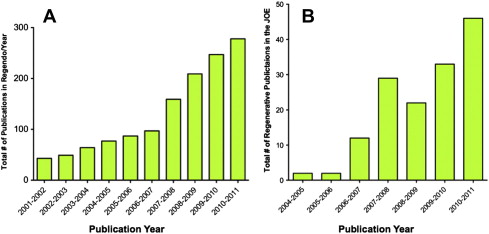
The American Association of Endodontists (AAE) and the AAE Foundation recently released a request for proposals for clinical research into regenerative endodontic treatment with up to $2.5 million in funding for the project. Aside from these future high-level studies, the current case studies offer the benefit of being performed in patients and thus provide a higher level of evidence than animal studies or in vitro studies.
Commitment to regenerative endodontics
Regenerative endodontics is a high priority for the specialty of endodontics. The number of publications in peer-reviewed journals related to regenerative endodontics has increased greatly in recent years ( Fig. 1 ). Organized endodontics is committed to identifying evidence-based methodologies for these procedures. The next logical phase of regenerative endodontic research will be prospective randomized clinical trials.
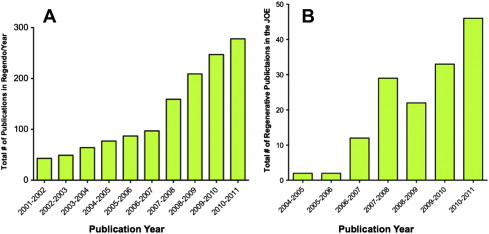
The American Association of Endodontists (AAE) and the AAE Foundation recently released a request for proposals for clinical research into regenerative endodontic treatment with up to $2.5 million in funding for the project. Aside from these future high-level studies, the current case studies offer the benefit of being performed in patients and thus provide a higher level of evidence than animal studies or in vitro studies.
Trauma literature
Much of the basis for these case studies has rudiments in the trauma literature. Revascularization can occur but requires a particular set of circumstances that, until recently, were thought to be exclusive to the avulsed immature permanent tooth. First, the exclusion of bacteria is the key factor in the success of this process. Second, the combination of an open apex and a short root allows the ingrowth of well-vascularized tissue. Third, the devitalized uninfected pulp is also thought to act as a scaffold for the ingress of apical tissue. Successful revascularization of the pulp leads to the maturation of the root and deposition of hard tissue within the root, both of which improve the probability of long-term retention of the tooth.
The notion then emerged that, if the pulp could be adequately disinfected, a blood clot matrix induced, and a bacteria-tight seal restored in a necrotic infected immature tooth, then, by extrapolation, revascularization should be expected to occur as in the devitalized, uninfected, avulsed, immature permanent tooth.
Terminology
Revascularization, revitalization, and regeneration have all been used to describe the outcome of these procedures. There remains some disagreement in the terminology. In vivo evidence suggests that it is unlikely that a functional pulp-dentin complex is being regenerating with current protocols. Wang and colleagues noted that the tissue that is responsible for increased root thickness in their study was cementum and not dentin. As more is learned about the tissues that are present through both molecular methods and human histology, it will become possible to better define what it is being accomplished. This article unifies all 3 terms under the broader category of REPs and regenerative endodontic outcomes.
Goals of REPs
The goal of regenerative therapies is to regenerate a fully functional pulp-dentin complex that fosters continued root development for immature teeth, and prevents or resolves apical periodontitis. As in traditional endodontic therapy, outcomes have various criteria for success. The degree of success of REPs is largely measured by the extent to which it is possible to attain primary, secondary, and tertiary goals.
The primary goals are the elimination of symptoms and the evidence of bony healing. Secondary goals (which are desirable but perhaps not essential) include increased root wall thickness and/or increased root length. A tertiary goal (which, if achieved, indicates a high level of success) is a positive response to vitality testing. Histologic confirmation of dental pulp with an intact odontoblastic layer and restoration of a functional pulp is the pinnacle of regenerative treatment goals.
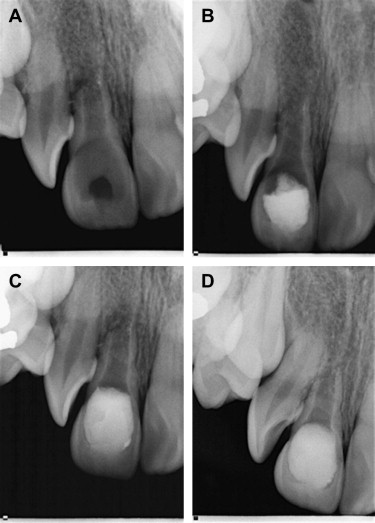
Regardless of this ultimate outcome, it is often beneficial to consider REPs if only to have a tooth act as a space maintainer until a suitable restorative option is available. In some cases, the achievement of the primary goals is all that is necessary to deem the procedure a success. REPs may provide hope for those teeth with unfavorable prognoses ( Fig. 2 ).

Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


