Improvement in the results of correction of maxillofacial deformities by orthodontic treatment and orthognathic surgery has been dramatic over the past 30 years. The vast majority of treated patients show improved occlusion and esthetics, but it must be stressed that unless symmetry and balance characterize relevant muscles, the predictability of long-term changes in bone and soft tissue is variable, irrespective of the method used to move bones.
In cleft lip/palate (CLP) patients, aberrations in craniofacial morphology and growth have often been attributed to the technique and timing of primary lip and palate surgery. This attribution overlooks the influence of inherent growth tendencies, such as those resulting from variation in cranial base morphology, irrespective of the technique and timing of cleft surgery ( Fig. 88-1 ). Thus, when evaluating the results of surgical treatment in CLP patients, cranial base morphology should be included as one influential factor in dental/skeletal relationships.
Orthognathic surgery is often thought of only in terms of osteotomies. This perception probably arises because orthognathic surgery is unsurpassed in its applicability for correcting certain dentofacial deformities, such as vertical maxillary excess. Orthognathic surgery, though, is much broader than just osteotomies; it is a constellation of procedures that permits differential alteration and repositioning of bone, cartilage, muscle, teeth, gingiva, mucosa, and skin. Development of the midface is both hierarchical and integrated. The development of muscles precedes that of bones. The bones of the midface develop under the influence and at the direction of the enveloping muscles. In other words, muscles are shaping bones. A good example of muscles controlling bones is what happens in utero with cleft lip and palate. The distortion of the bones is already present when the baby is born. This is muscle distortion of bone, and in the case of cleft lip and palate, all of the cheek muscles and lip-nose muscles, acting through the breach, combine to distort the face. Early orthognathic surgery is muscle surgery. In this case, early orthognathic surgery seeks to achieve differential alteration in repositioning of cartilage, muscle, mucosa, gingiva, and skin. Once this has been accomplished, the bones will follow.
Accordingly, the cleft surgeon obviously must master the surgical techniques that best meet the needs of the patient at given ages. Moreover, he or she must also be a physiologist, particularly in knowing and using, to the benefit of patients, all of the details of the development of the facial skeleton, the quality of which, at the end of growth, essentially determines the quality of the surgical result and the ultimate need for and execution of orthognathic surgery in patients with cleft lip and palate.
Etiopathogenesis, Causative Factors, and Pathologic Anatomy
The major subdivisions of the human skull are the desmocranium (skull proper), chondrocranium (cartilaginous base of the skull and the sense capsules), and viscerocranium (maxillofacial and mandibular skeleton). The major functional skeletal units of the maxilla consist of dermal (membrane) bones that develop directly (intramembranously) in embryonic mesenchyme without the involvement of a cartilaginous model or precursor.
The osteoblasts and osteocytes that form the maxillofacial skeleton do not arise in situ but, rather, arise from cells that are initially associated with the developing neural tube. These cells, the neural crest cells, must therefore migrate to their final site in the developing embryo.
During the formation of the primary neural axis, a special population of cells, the neural crest cells, can be found in the folds of the developing neural tube. As the neural folds close over, these cells break free of the neuroepithelium, undergo a morphologic transformation from an epithelial organization (as part of the continuous sheet of cells of the neural tube) to a mesenchymal (loose meshwork) organization, and begin to migrate from the neural tube toward the future facial primordia.
The skeletogenic capability of the neural crest has been determined both by following labeled cells in vivo as they differentiate into chondroblasts, osteoblasts, and odontoblasts (dentine is also a neural crest derivative) and by culturing or grafting neural crest–derived mesenchyme along with an appropriate inducer of chondrogenesis or osteogenesis. How future skeletogenic neural crest cells localize appropriately along the developing neural tube remains largely unknown.
Although the human maxillofacial skeleton lies completely within the territory of neural crest–derived structures, much of the skull roof does not; it arises from mesodermally derived mesenchyme that forms in situ.
Neither neural crest– nor mesodermally derived mesenchyme self-differentiates into osteoblasts, chondroblasts, or odontoblasts; rather, they have to be induced to differentiate. These particular inductions are known as epithelial–mesenchymal interactions, because the inducing tissue is an epithelium and the responding tissue is mesenchyme. Most skeletogenic inductions are matrix-mediated and are therefore time dependent.
Once differentiation has been initiated by one or more epithelial–mesenchymal interactions, other factors regulate subsequent development and growth of the maxillofacial complex. Individual elements or regions do not develop and grow at the same rate. Such regional differences imply that development and growth are not regulated globally. Even factors such as hormones that circulate throughout the developing embryo can only act upon cells that possess the appropriate receptors.
Evidence for differences in local environments within the embryo comes from the concept of functional matrices. During late embryonic life, the development and growth of the cranium is influenced by the expanding and growing brain, that of the mandible and palate by the growing tongue, and the maxillofacial region by the expanding eye and growing nasal septum. Postnatally, these functional matrices are replaced by growing muscles, ligaments, and erupting teeth. The same elements of the head and face are therefore subject to regulation by different factors at different times during pre- and postnatal development.
Adjacent skeletal units, even though controlled by different functional matrices, influence one another because they are adjacent, begin their development at different times, develop and grow at different rates, and respond to different controls. Thus, the cranium grows fast and early to keep pace with the early and rapidly growing brain while the mandible and maxilla grow slowly and late, their rates being more in tune with general somatic growth than with brain growth. The influence of cranial growth on facial growth is because of timing, structure, and function. Because the cranium grows to keep pace with the rapid and early growth of the brain, it especially influences growth of the upper third of the face, which, accordingly, displays the fastest rate of facial growth and complete growth first (around 12 years of age). The remainder of the maxillofacial region is less affected by brain growth and, accordingly, grows more slowly and for a longer time, growth not being completed until the late teens or early twenties.
Diagnosis, Reconstructive Goals, and Treatment
The use of cephalometrics has become a specialized part of the evaluation of patients with craniofacial deformities, permitting the clinician to systematically describe discrepancies in the maxilla, mandible, dentoalveolus, and soft tissue mask of the face. The relationships of points, lines, and angles measured on the conventional cephalometric radiograph are useful diagnostic aids to the extent that they are based on statistical means from which deviations have clinical significance. Conventional cephalometry does not stress pathophysiologic principles, nor do conventional cephalometric analyses include cranial analysis, because the conventional lateral cephalometric radiograph does not include an image of all of the cranial osseous structures.
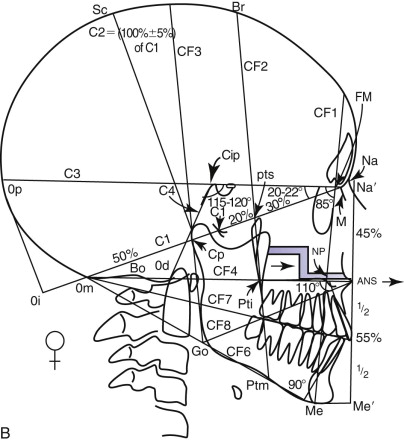
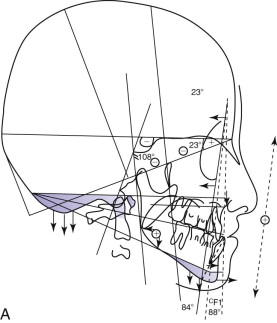
Delaire has advocated the use of the architectural and structural craniofacial analysis that was conceived to account for all of the architectural and structural craniofacial information available on the lateral skull radiograph. This includes all of the head and its associated soft tissue: nose, lips, scalp, larynx, pharynx, and the neck, including the first three or four cervical vertebrae ( Fig. 88-2 ). All of these structures can be assessed, to a greater or lesser degree, in a cephalic context by the use of the architectural and structural craniofacial analysis of Delaire. The analysis is composed of two distinct phases. The lines of craniofacial balance, which allow us to view objectively and quantify the variations of equilibrium between the patient being examined and the “normal” patient in his or her peer group, represents the “architectural analysis” ( Fig. 88-3, A ). Direct study of the cephalometric radiograph of the osseous structures—their dimensions; degree of ossification, trabeculation, and cortication (external contour); and neighboring soft tissues (both superficial and deep)—represents the “structural analysis” ( Fig. 88-3, B ).
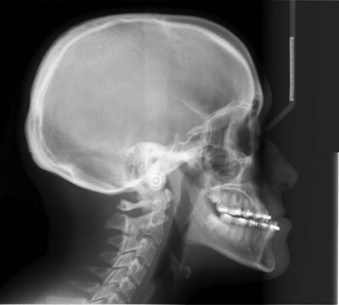
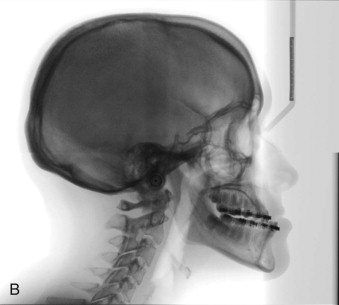
The analysis requires a lateral cephalometric radiograph of high quality, showing clearly the hard and soft tissues of the whole head and neck region. It should be taken with the patient staring into infinity, the mandible closed in centric relation, and the lips in repose (see Fig. 88-2 ).
There are six operations which constitute the basic skill set necessary to effectively manage patients with cleft lip and palate.
- 1
Primary functional cheilorhinoplasty and primary veloplasty
- 2
Palatoplasty
- 3
Alveolar bone graft
- 4
Maxillary osteotomy with secondary functional cheilorhinoplasty
- 5
Mandibular osteotomy
- 6
Functional genioplasty
Primary Functional Cheilorhinoplasty and Primary Veloplasty
Surgical correction of cleft lip remains an elusive problem, principally because the fundamental surgical problem is not clearly conceptualized. This failure to accurately define the problem is attributable to the fact that the relevant anatomy is complex, poorly understood, and frequently erroneously described. Meaningful correction of cleft lip can be achieved only when the surgeon is fully appreciative of both normal and pathologic spatial relationships and functions of the anatomic elements, particularly the muscular elements that cause the deformity. The treatment goal, therefore, is to obtain anatomic functional balance between the soft tissues and the skeleton.
To reestablish a “normal” situation and dimension of the mucocutaneous elements in primary closure of labiomaxillary clefts, it is necessary to achieve good position of the skin of the floor and sill of the nostril, obtain good height of the skin of the cleft side, re-create good form and continuity of the white roll of the lip, establish equal vermilion height on both sides of the cleft and on both sides of the middle of the lip, and excise abnormal mucosa from both sides of the cleft.
To achieve good positioning of the skin of the floor and the sill of the nostril, it is absolutely imperative to respect the cutaneous areas and their individual characteristics relative to the skin of the lip. By definition, this rules out all those incisions that intrude on these areas, notably, those that enter the nostril, curving either inside or outside of the ala and extending to the columellar base. This also rules out all cutaneous excision in the upper part of the lip. The best incisions in the upper part of the lip, therefore, are those that pass exactly between the skin of the nose and that of the lip, and that stop laterally at the external border of the alar base and medially below the border of the columella ( Fig. 88-4 ).
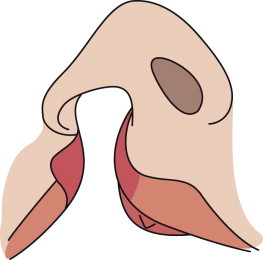
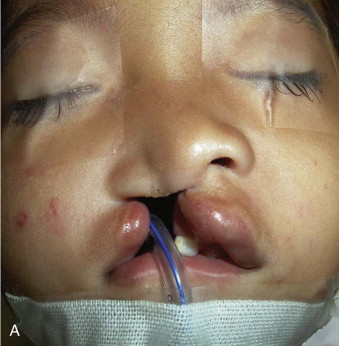
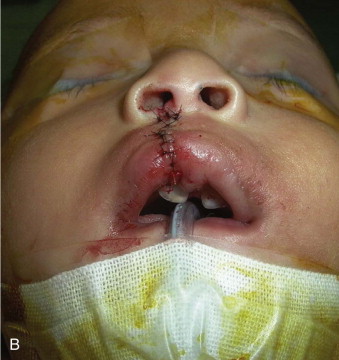
Accordingly, at the end of the primary lip operation, the surgeon must have achieved (1) a straight nasal septum positioned in the facial midline, (2) symmetrical reconstruction of the nasolabial muscles, (3) absence of vestibular oral nasal communication, and (4) a functional, patent nostril on the cleft side ( Fig. 88-5 ).
Palatoplasty
Velopalatine clefts are associated with divided, separated, displaced, hypotrophic, and sometimes hypoplastic muscles of the soft palate. The tongue and floor of the mouth are abnormally low, and the pterygoid processes are displaced laterally. The palatoglossus muscles are retracted laterally, and the levator veli palatini and palatopharyngeus muscles are displaced both laterally and superiorly. Because of the low position of the tongue, the tip can engage the posterior part of the cleft, thus exaggerating the lateral displacement of the pterygoid muscles and widening of the cleft.
The unwanted effects of either poor or incomplete reconstruction of the soft palate include hypotony, hypotrophy, and retraction of the soft palate and its anterior pillar, which lead to atrophy and sclerosis. As a consequence of an unfavorable tongue position, there is a tendency to mandibular protrusion and maxillary retrusion. Good reconstruction of the soft palate, therefore, should reconstitute the posterosuperior parts of the soft palate, particularly the levator veli palatini muscle. This reconstruction permits the soft palate to assume relatively normal functions of phonation, deglutition, and tongue position, all of which optimize skeletal morphogenesis in an orthognathic manner.
Whether the global deformity is due to true hypoplasia, hypofunction, and attendant hypodevelopment or a combination of both, the principal surgical goal is the same: to establish good function through careful muscle reconstruction, which, in turn, will permit optimum subsequent growth and development of the facial skeleton. This principle is important, both in primary and secondary cleft corrections, because good function is prerequisite to good facial esthetics.
There are three rather distinct regions of mucoperiosteum that cover the palate ( Fig. 88-6 ).
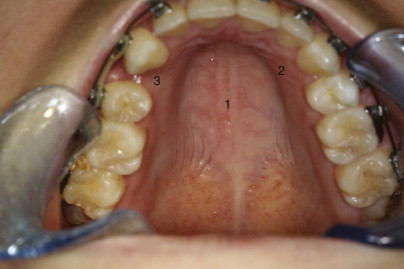
It is the thick maxillary mucoperiosteum, so important in the transverse and vertical growth of the palate, with which the surgeon must take special care in surgery of the hard palate. In total bilateral cleft palate, it is the palatine part that is missing, but the gingival and maxillary regions are usually, essentially normal. This explains why in unoperated bilateral cleft palate, with the exception of the vomer and the palatine shelves themselves, development of the palatal vault is almost normal. In classic palatorrhaphy techniques, large widely undermined flaps of the maxillary region are swung medially to cover the defect of the palatine part, thus leaving bilateral donor areas of exposed bone that eventually undergo wound contraction and scarring. From its new position, the displaced maxillary mucoperiosteum cannot play its important role in transverse and vertical growth of the palatal vault. Furthermore, because this tissue is thick (rather than the thin palatine mucoperiosteum that is normally situated here), there is a “filling in” effect, which further compromises the depth of the palatal vault. The nasal fossae, normally under the influence of the palatine region of mucoperiosteum, do not fully develop their transverse dimension, which, in turn, can affect nasal airway patency. In classic palatorrhaphy, it is also common to obtain the nasal layer of the two-layer closure by the use of flaps of vomerine mucosa. This establishes an insufficient vertical dimension of the maxilla and, therefore, also of the nasal fossae; the net result favors a vertically deficient, retrodisplaced maxilla and overclosure of the mandible.
From these observations, it seems prudent for the surgeon to attempt to avoid medial transposition of maxillary mucoperiosteal flaps, exposed areas of bone at completion of the operation, and fixation of the vomer to the palate by use of vomerine mucosa to establish the nasal plane in two-plane closures. Staging of the surgical procedures based on variation of the individual case should be planned such that it is possible to respect the principles of anatomy and physiology of the three regions of mucoperiosteum that cover the palate.
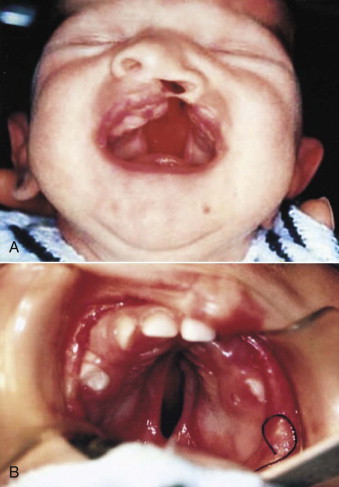
In complete cleft lip and palate, the soft palate is closed at the same time as the cleft lip at about 3 to 5 months of age. The surgical goal is to establish both continuity and function of the muscles of the soft palate. Adequate length of the soft palate can be achieved without either complex, multiple Z -plasties or microsurgical techniques. Incisions are made on the margins of the cleft of the soft palate, slightly favoring the nasal side. To obtain the best exposure of the levator muscle, which is retracted toward the nasal side, a small triangle of mucosa is excised from the nasal surface of the divided velum on both sides. Reconstitution of the levator veli palate, as well as the palatopharyngeus and palatoglossus muscles, is prerequisite to obtaining adequate soft palate length. Mucosal incisions are made on both sides to allow exposure of the hamular process, which is not fractured by design. Adequate soft tissue mobilization can easily be realized by meticulous muscle dissection. The tensor palati and superficial portion of the palatopharyngeus muscles are freed from the posterior border of the palate so that their orientation from longitudinal to transverse can be accomplished. Muscle reconstruction, including the palatoglossus, establishes a functional sphincter of the soft palate. No vomer flap is employed.
Following closure of the soft palate and the cleft lip, there is now function both anteriorly and posteriorly, which causes the distance between the hamular processes, tuberosities, and the divided hard palate to dramatically diminish by the age of about 12 months. At this time, the residual hard palate cleft can be closed, very often without the use of lateral palatal incisions ( Fig. 88-7 ). Again, no vomer flap is used because the nasal side of the cleft is closed using nasal mucosa situated below the inferior border of the vomer. Isolated cleft palate, both hard and soft, is treated at about 9 months of age. The same fundamental principles are employed in revision surgery to obtain lengthening and improved function of the soft palate.
Alveolar Bone Graft
When the timing of alveolar bone grafting is based on the eruptive progress of the permanent maxillary canine teeth, the surgical procedure is, by definition, delayed until the late mixed dentition. This treatment strategy overlooks both the crown length and the periodontal condition of the maxillary permanent incisors adjacent to the cleft defect.
The goals of alveolar bone grafts are
- •
closure of vestibular and palatal oral nasal fistulae.
- •
to provide adequate bone stock for the permanent maxillary central incisor, lateral incisor, and canine teeth.
- •
to establish the nasal skeletal base.
- •
to provide a suitable bony architecture on which to perform symmetrical nasolabial muscle reconstruction.
- •
to establish a functional floor of nose and nasal airway on the cleft side.
- •
to provide adequate bone stock for placement of an osseointegrated dental implant.
Grafting is usually performed when the maxillary permanent incisor teeth are visible in the mouth but not yet fully erupted. Simultaneous secondary functional cheilorhinoplasty is performed if there is muscular dysfunction of the lip as determined by deviation of the nasal septum to the noncleft side, presence of vestibular oral nasal fistula, and/or inability of the child to symmetrically project the lips. Total palatal reconstruction is carried out if there are palatal fistulae or distorted palatal anatomy resulting from inaccurate and inadequate primary surgery. Except in the most severe cases, the bone graft procedure is carried out prior to orthodontic expansion, which is usually initiated about 8 to 12 weeks after grafting. This allows the arch expansion to be accomplished concomitant with a type of distraction osteogenesis, accompanied by appropriate palatal mucosa expansion.
The procedure begins with infiltration of 2% lidocaine with 1/100,000 epinephrine into both the palatal mucoperiosteum and the vestibular sulcus on both sides of the cleft. Full-thickness mucoperiosteal palatal flaps are developed on both sides of the palatal aspect of the cleft using incisions extending from the tuberosity region, around the cervical portion of the teeth to meet at the cleft in the anterior maxilla. This broad dissection allows accurate definition of the palatal-nasal defect and its contiguous nasal mucosa.
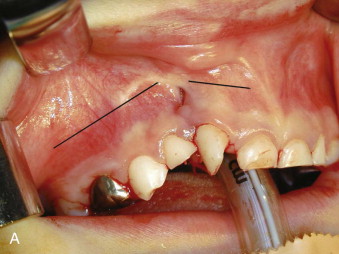
A horizontal incision is then made on the vestibular side about 5 mm above the attached gingiva and extended medially to a point just short of the margin of the fistula if present, but, in any case, just short of the bony margin of the cleft defect ( Fig. 88-8, A ). The lateral aspect of the maxilla is widely exposed to include the lateral aspect of the cleft from the existing alveolar bone margin to a point on the lateral aspect of the pyriform aperture equal in height to that of the infraorbital foramen. This dissection is carried on the inner aspect of the maxilla in the nasal aspect such that a vertical sheet of nasal mucosa is developed that is continuous with the palatal dissection already carried out. On the medial aspect of the cleft, a similar horizontal vestibular incision is made that permits complete exposure of the lateral nasal margin of the noncleft nostril, the anterior nasal spine, and the medial margin of the cleft alveolus. The nasal mucosa on the medial aspect of the cleft is then developed such that this dissection too is continuous with the palatal dissection. A small vertical incision then is made in the attached gingiva so that the entire cleft alveolar defect is now clearly visible.
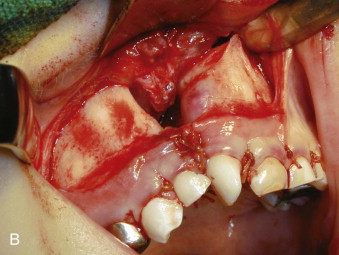
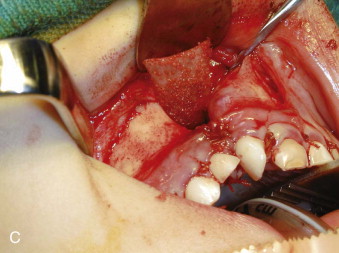

The palatal mucosa is reconstructed, from posterior to anterior, using interrupted mattress sutures to ensure as perfect palatal mucosal anatomy as possible. The reconstructed palatal mucosa is left as a large single palatal flap until the nasal reconstruction is completed. From the vestibular side, the nasal mucosa is now reconstructed such that the height of the nasal floor is equal to or slightly superior to that on the noncleft side. This is a very important technical point, because unless there is adequate space for bone between the palatal and nasal layers, long-term bone stock quantity will be insufficient. Part of the nasal mucosal suturing is carried out through the access created by delaying the final suturing of the palate. Meticulous attention to detail in suturing the nasal mucosa is important so that a smooth and even mucosal layer presents to what will be the roof of the bone graft ( Fig. 88-8, B ).
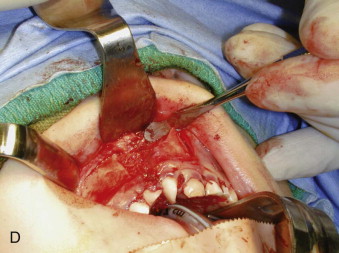
A thin piece of cortical bone is fashioned to snugly fit in the nostril defect, dividing the graft from the nasal mucosa at a height slightly above the nostril floor on the noncleft side. The cortical side of this piece of bone is placed against the nasal mucosa, the medullary side thus facing the graft ( Fig. 88-8, C ). A nasopharyngeal airway is now placed in the nose to ensure a patent and functional airway while the particulate marrow is being placed. Particulate marrow is packed into the defect with pressure. As much bone as possible is placed into the confines of the defect, paying particular attention to the alveolar margin of the cleft in the region of the erupting teeth ( Fig. 88-8, D ).
The vertical incision in the attached gingiva is closed such that the reconstructed gingiva is resting on newly grafted bone. The palatal flap is then returned to its ideal position and sutured in place around the necks of the teeth and at the anterior aspect over the graft. The vestibular horizontal incisions are closed so that the resulting vestibular depth is not diminished. The procedure is very similar when carried out in conjunction with secondary functional cheilorhinoplasty. At the end of the operation, only palatal mucosa is on the palate, only nasal mucosa forms the nasal floor, attached gingiva (not alveolar mucosa or any other foreign tissue) covers the teeth and the graft at the alveolar margin, and the maxillary vestibule is lined only by alveolar mucosa. This strict anatomical geography is critical to the long-term success of the operation. No splints are used, but the patient is placed on a soft diet for about 1 week after surgery.
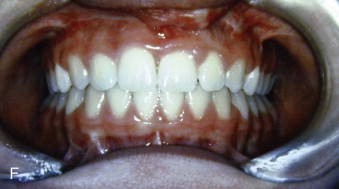
Alveolar bone grafts that are performed just prior to or very soon after the beginning of eruption of the maxillary permanent central incisor contralateral to the cleft provide good nasal floor symmetry ( Fig. 88-8, E ) and almost normal symmetry of clinical crown length of the two erupted permanent maxillary central incisors ( Fig. 88-8, F ).
Maxillary Osteotomy
The maxillary osteotomy of preference is usually the Le Fort I type, but the clinician should be aware of the applicability of the modified Le Fort III or maxillomalar osteotomy when there is deficiency in the anterior projection of the malar aspect of the face.
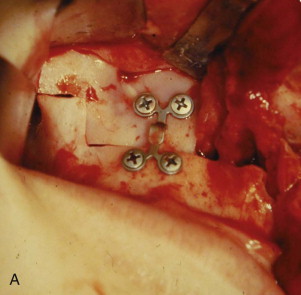
The approach to the maxilla for the Le Fort I osteotomy is similar to that described by Bell, with several modifications. The horizontal incision is kept very low such that only a few millimeters of unattached mucosa remain on the inferior segment. This modification, when combined with very wide subperiosteal exposure, particularly on the cleft side, allows maximum advancement of soft tissue toward the midline. We invariably use a superior step in the zygomatic region of the maxilla to enhance the accuracy of intraoral spatial orientation, to provide solid bone for application of rigid fixation devices, and to maximize bony contact of the bony fragments ( Fig. 88-9, A ). We do not use a chisel to separate the maxilla from the pterygoid plates; rather, we employ a Tessier spreader to exert downward pressure on the posterior aspect of the maxilla, which results in a clean and easy pterygomaxillary separation. The maxilla is then oriented on the mandible using an intermaxillary occlusal splint, which is initially ligated to the maxillary arch. Contoured mini-plates are used to fixate the maxilla in its planned (slightly overcorrected) position. The reverse step in the osteotomy design permits use of posterior wire osseous fixation, which “locks in” the bony segments and reduces the cost of the fixation ( Fig. 88-9, B ).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses