Fig. 13.1
Healthy gingiva, gingivitis, and periodontitis in the oral cavity. (a) Healthy gingiva. The pale-pink, stippled gingiva is attached tightly to clean teeth. The gingival sulcus is indicated (arrow). (b) Gingivitis. The gingiva is red, especially at the free gingival margins (upper arrow) and plaque is present (lower arrow). Patients with moderate to severe gingivitis (illustrated in this slide) complain that their gums bleed on toothbrushing. (c) Periodontitis: The gingiva has receded and the cementum has been abraided, exposing the dentinal surface of the tooth root (recession, arrow). The gingiva is inflamed (red) and the sulcus is deepened. Periodontal pockets have formed between the exposed dentin and the inflamed (red) gingival
13.1.1 Gingivitis and Chronic Periodontitis in Humans and Animals
The present-day understanding of how the commensal microbiota causes gingivitis began with a human study of experimental gingivitis by Harald Löe and his colleagues in Denmark in 1965. Adults with no periodontal attachment loss are selected, and their teeth are thoroughly cleaned for a few days. They then abstain from oral hygiene procedures for 3 weeks. A microbial biofilm (plaque) adheres to teeth, and an inflammatory exudate appears at the sulcus, the Gingival Crevicular Fluid (GCF; Table 13.1). This fluid is derived from serum, blood plasma in which fibrin clot formation is prevented by plasmin activation (Sect. 13.2.3). After a week, gingivitis appears and extends to all teeth within about 3 weeks. The severity of gingivitis is measured by gingival index (Tables 13.2) averaged across the sites described above for attachment level. Dogs develop periodontal disease as in humans, except that a hard diet cleans the teeth instead of oral hygiene. Changing dogs from hard to soft diets causes biofilm accumulation and gingival inflammation, which progress to periodontitis. Other animal models are less satisfactory. Gingivitis and periodontitis are induced in non-human primates only if silk ligatures are placed at the gingival margins. In rodents, periodontitis only develops if the endogenous commensal microbiota is depressed by antibiotics and the oral cavity monoinfected with an oral bacterial species. Hair and bedding become trapped in gingival pockets and may enhance periodontitis development. Gingival inflammation is minimal.
Table 13.2
The gingival index
|
Marginal gingiva
|
Score
|
|---|---|
|
Pink and stippleda
|
0
|
|
Red and edematousb
|
1
|
|
Bleedsc within 10 s
|
2
|
|
Bleedsc immediately
|
3
|
Table 13.1
Interstitial and gingival crevicular fluid composition
|
ISF
|
GCF
|
|---|---|
|
Transudate
|
Exudate
|
|
No proteins
|
Rich in proteins
|
|
No neutrophils
|
Many neutrophils
|
Another bacterium that contributes to commensal biofilms is Eikenella corrodens, a gram negative, small rod (Fig. 13.2c). E. corrodens grows in a healthy oral cavity by reducing nitrate in saliva to nitrite (Sects. 1.3.2 and 12.1.3). It is an important contributor to bite wound infections and is also the major known producer of lysine decarboxylase, which converts lysine to cadaverine and carbon dioxide (Fig. 13.4). Lysine is a nutritionally essential amino acid, whereas cadaverine is not. In humans, E. corrodens and lysine decarboxylase activity increase markedly in teeth-adherent biofilms during the first week of experimental human gingivitis.
Lysine decarboxylase may play a critical role in initiating gingivitis because basal cells of the epithelial attachment at the base of gingival sulci must proliferate continuously to maintain a basal lamina seal (Sect. 5.2.3). By starving these epithelial cells of lysine, their proliferation and ability to seal the base of a sulcus are impaired. This means that commensal microbial products could better access the gingiva where they induce inflammation and GCF exudation (Sect. 13.2.1). E. corrodens is a common cause of dog bite infections, and if dogs are fed a soft diet to accumulate biofilm faster (Sect. 13.1.2), immunization with purified lysine decarboxylase could induce antibodies that slow gingivitis development. The GCF is a richer substrate for bacterial growth than saliva, and it promotes the development of a predominantly gram negative successor and climax microbiota in gingival sulci (Fig. 13.3b). Individual bacteria contributing to the successor microbiota constantly enter the oral cavity from the environment. Some coaggregate within the biofilms and generate autoinducer-2 levels that promote their growth in GCF at the expense of the commensal microbiota. It is generally accepted that the successor microbiota are mostly responsible for gingivitis and chronic periodontitis.
The metabolism of the successor microbiota is asaccharolytic. Its various bacteria release ammonia from all amino acids and sulfides that cause oral malodor from cysteine and methionine amino acids (Sect. 1.3.2). When albumin, citrate, and pyrophosphate in the GCF are hydrolyzed, calcium ions (Sect. 9.1.4) become free to precipitate with phosphate ions and form calculus. Calculus protects the biofilm at the base of a gingival sulcus, promoting persistent inflammation that impairs the viability of the junctional epithelial attachment (Sect. 13.4.1). Salivary gland loss or malfunction, or persistent mouth-breathing or tobacco smoking, dry up the mouth and enhance bacterial colonization and disease (Section 12.1.3). In periodontitis, bacterial products pass from gingival capillaries into the systemic circulation and promote inflammation that worsens some preexisting diseases, notably cardiovascular disease, and adult-onset diabetes. Periodontitis also predisposes to bacterial endocarditis (bacteria attaching to heart valves) and low birth-weight babies.
13.1.2 Microbiota of Gingivitis and Chronic Periodontitis in Man
Because animals require sedation before sampling the oral cavity, most studies have been of the human oral microbiota. These studies indicate that, in a healthy oral cavity, bacteria attach to the oral mucosa and are continually shed into saliva (Sect. 12.1.5). This commensal microbiota is present in saliva (Sect 12.1.5) and loosely adherent to the oral mucosa. It is mostly composed of viridans streptococci and Actinomyces species (Fig. 13.2a, b). These bacteria hydrolyze sialic acid and glycans from mucins and glycoproteins on the mucosal surface or in saliva (Sect. 12.3.1), and they grow by metabolizing the glycans to carbon dioxide and water (respiration, Sect. 1.3.1). They also attach to teeth surfaces where mutualistic interactions, characterized by luxuriant mixed culture growth on saliva alone in vitro or in vivo, generate a biofilm. These interactions are called quorum sensing, and they are mediated by 4,5-dihydroxy-2,3-pentanedione (DPD; Fig. 13.3a).
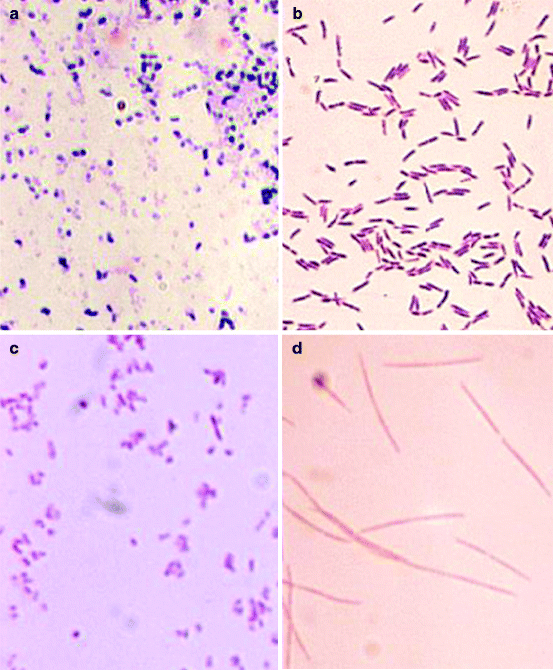
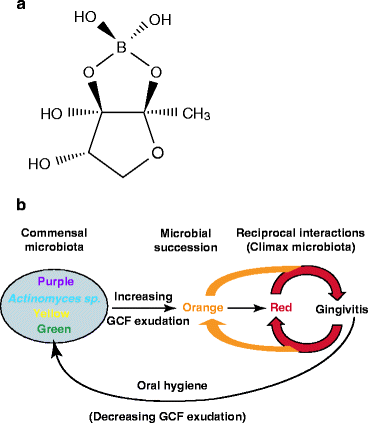
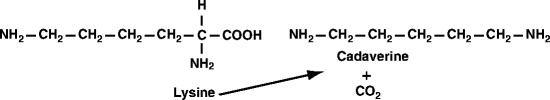

Fig. 13.2
Common bacteria of plaque. Slides show typical gram stain for: (a) viridans streptococci; (b) Actinomyces naeslundii, previously A. viscosus; (c) Eikenella corrodens, and (d) Fusobacterium nucleatum (From Public Health Image Library (PHIL) at the CDC – Bacteria Site: http://phil.cdc.gov/phil/home.asp.

Fig. 13.3
Bacterial colonization and succession in gingivitis. (a) Structure of autoinducer-2. All possible configurations of the OH groups occur spontaneously in solution due to boron-catalyzed hydrolysis and rearrangement. Different bacterial species have receptors that only recognize one or a limited number of these configurations (b) Bacterial complexes. The commensal bacteria attach to teeth as distinct complexes within a biofilm (plaque). The most prominent complexes are Actinomyces spp. (blue), Streptococcus spp. (yellow) and a mixture of Capnocytophaga spp. with E. corrodens (green). The successor microbiota attaches to these commensal biofilms as complexes of Fusobacteria spp. with many other gram negative bacteria (orange) and a climax complex of just three species: Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia (red) (a: Public domain image: http://en.wikipedia.org/wiki/Autoinducer-2; b: Modified from Fig. 1. in Socransky and Haffajee, “Periodontal microbial ecology.” Periodontology 2000, 38:135–187, Blackwell Munksgaard 2005)

Fig. 13.4
Reaction catalyzed by lysine decarboxylase
DPD is also called autoinducer-2 (AI-2; AI-1 is another, less common inducer). AI-2 is derived from S-ribosyl homocysteine which is hydrolyzed to homocysteine and 1-deoxy-3-dehydro-d-ribulose in bacteria. The d-ribulose derivative is extruded as a boric acid ester (boron is a trace element, Chap. 1), which spontaneously rearranges into numerous isomers that, together, comprise AI-2. Each isomer binds with a structurally and functionally distinct receptor. Thus, quorum sensing through AI-2 secretion activates the expression of genes required for growth by mutualistic interactions within biofilms. For example, coaggregation with an actinomyces species enables a streptococcus species to switch from quiescence to active growth when arginine is scarce as it is in saliva.
13.1.3 Drugs to Prevent Gingivitis
In addition to toothbrushes and dental floss for mechanical oral hygiene (Sect. 13.5.1), antiseptic mouthwashes exert chemical control of the microbiota. The most commonly sold mouthwashes in the United States are made from mixtures of volatile aromatic compounds from plants. Different mixtures of these plant compounds possess a unique scent or essence and are commonly called “essential oil” mixtures. They have antiseptic properties that control the successor microbiota, but a need for 15–20% alcohol to maintain “essential oil” solubility has been linked to an increased risk of oral cancer in long-time daily human users. Less popular mouthwashes contain an antiseptic called chlorhexidine (Peridex) or a detergent that interferes with bacterial biofilm aggregation called delpolminol (Decapinol). Long-term use of all of these mouthwashes interferes with taste and probiosis, protection by the commensal microbiota from pathogenic organisms.
The next generation of drugs to supplement oral hygiene may interfere more specifically with one of four actions: (a) initial stages of bacterial attachment to teeth surfaces; (b) coaggregation of successor bacteria into a biofilm; (c) quorum sensing; and (d) lysine decarboxylase impairment of the epithelial attachment barrier. Recent studies by the author suggest that immunological inhibition of E. corrodens lysine decarboxylase in beagle dogs, or chemical inhibition of this enzyme in humans, retards gingivitis development and therefore the eventual development of chronic periodontitis.
Summary
Periodontal disease describes a mixture of diseases in which periodontal attachment is destroyed. It may be chronic or aggressive, and localized or generalized. Chronic periodontitis first appears as gingival inflammation (gingivitis) accompanied by a teeth adherent microbial biofilm (plaque) and calculus. Bacteria are always present in the oral cavity. They grow on mucosal surfaces, in saliva, and adhere to teeth as biofilms within which quorum sensing generates chemicals that activate mutualistic interactions leading to luxuriant mixed culture growth. Eikenella corrodens in the commensal microbiota is a major source of lysine decarboxylase, which depletes the interstitial fluid of lysine necessary to maintain an intact epithelial barrier at the base of gingival sulci. Loss of this barrier likely increases exposure of the gingiva to individual members of a gram negative, successor microbiota that induce an inflammatory exudate derived from blood plasma, the gingival crevicular fluid, which is richer in bacterial substrates than saliva. The GCF promotes quorum sensing by the successor microbiota at the expense of the commensal microbiota and gingivitis appears. Current mouthwashes contain antiseptics or detergents that supplement oral hygiene but interfere with probiosis, protection of the oral cavity by the commensal microbiota. New drugs that maintain probiosis would inhibit bacterial attachment to teeth, quorum sensing, or lysine decarboxylase impairment of the epithelial barrier.
13.1.4 Mammalian Cells Recognize Prokaryotic Molecules
The successor microbiota is a major source of products containing conserved prokaryotic motifs, pathogen-associated molecular patterns (PAMPs). The best-known PAMPs are lipopolysaccharides of gram negative bacteria (Sect. 1.4.2). Lipopolysaccharides are recognized by pattern-recognition receptors (PRRs) known as toll-like receptors (TLRs) on the mammalian cell surface. [TLRs received their name from their homology to a protein encoded by a gene identified in 1985 from Drosophila flies. Mutations of this gene made the flies look so unusual, that German researchers spontaneously called out “toll,” their word for “amazing.”]. Other PAMPs are membrane permeable, for example, bacterial peptidoglycan fragments (Sect. 1.4.1), fatty acids hydrolyzed from lipopolysaccharides (Sect. 1.4.2), bacterial DNA fragments, and various other products of lysed bacteria. These agents are recognized by PRR proteins that are soluble in the cytosol and have an amino acid sequence homology to nucleotide-binding oligomerization domain (NOD) proteins. There are 23 such NOD-like receptor proteins (NLRs) in humans, some of which have alternative names derived from findings before this family and its functions were recognized. PAMP-bound TLRs mediate inflammation, whereas PAMP-bound NLRs mediate either inflammation or tissue destruction, depending on the state of the cell and type of molecule recognized (Sect. 13.4.1).
13.1.5 PAMPs Induce PRRs to Release Cytokines That Attract Leukocytes
Innate immunity to infections is mediated by many of the same enzymes that function in bone demineralization, i.e., acid-activated lysosomal enzymes (Sect. 10.1.1). These enzymes are brought to infected regions by leukocytes (neutrophils and macrophages). PAMP-bound PRRs release two endogenous ligands (proinflammatory cytokines) that attract and activate leukocytes from the cytosol of junctional epithelial cells and gingival fibroblasts. One important cytokine, interleukin-1 (IL-1), is released from the cell cytosol by ligand-bound PRRs causing changes in membrane associated proteins without involving the Golgi or secretory pathways.
IL-1 is stable, and it diffuses to and activates receptors (IL-1 receptors) on adjacent and distant cells. The IL-1 receptor of endothelial cells is especially important (Fig. 13.5a). In all cells, the IL-1 ligand bound to its receptor causes surface expression of a second cytokine, tumor necrosis factor-α (TNF-α). TNF-α was originally described as a factor that kills (causes necrosis of) mouse fibrosarcoma (cancer) cells but not normal mouse fibroblasts. TNF-α is released from the cell surface by an adamalysin protease, TNF alpha converting enzyme (TACE). TACE is also known as ADAM17, one of more than 40 cell surface bound adamalysins related to the ADAM-TS2 subfamily (Sect. 8.2.1). Unlike IL-1, TNF-α is unstable and can only activate TNF receptors on nearby cells. It usually produces responses that supplement those from IL-1 activation (Fig. 13.5b).
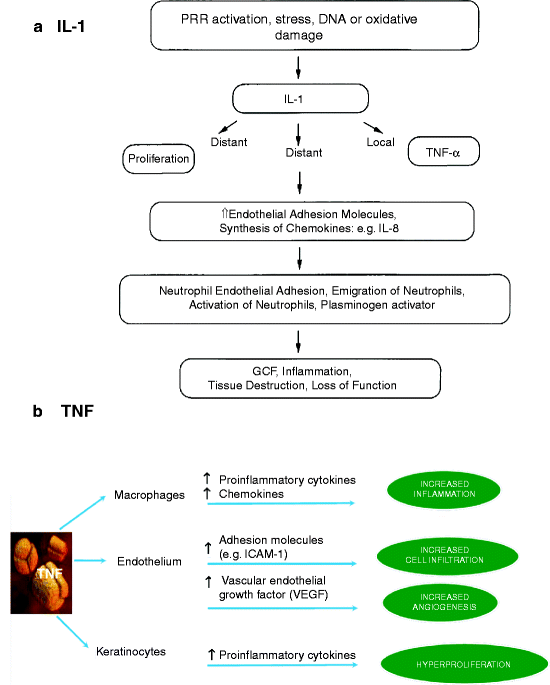

Fig. 13.5
How IL-1 and TNF-α cause inflammation. (a) IL-1 function. IL-1 induces the secretion of TNF-α to enhance its actions. The major effect of IL-1 is on capillary endothelial cells (see text). IL-1 causes keratinocytes and endothelial cells to proliferate and secrete other interleukins, especially IL-8, which acts like IL-1 on capillary endothelial cells (b) TNF-α function. TNF-α usually acts as a proinflammatory growth and survival factor (see text). Its induction of vascular endothelial growth factor (VEGF) promotes greater capillary proliferation and permeability. VEGF contains a cysteine knot domain like von Willebrand factor (Fig. 11.2) [a: based on Fig. 1 in Dinarello CA (2000) “Proinflammatory Cytokines.” Chest 118(2):503–508; b: Slightly modified Fig. 1 from Jackson JM (2007); TNF-α inhibitors, Dermatologic Therapy, 20:251–261]
IL-1 is released as two forms, IL-1α and IL-1β, encoded by separate genes that are only 28% homologous. The expressed polypeptides form a 12-stranded β-sheet around a central axis such that two sets of six β-sheets come together to form an antiparallel β-barrel (Fig. 13.6a). Similar β-barrel structures are present in α-amylase and many other proteins that recognize carbohydrates (Sect. 12.5.2). IL-1 is guided to its receptor by interacting first with glycans on the cell surface. The receptor-binding domain, the C-terminal domain (Fig. 13.6b), is correctly folded in IL-1α, but that of IL-1β must be cleaved from its N-terminal domain by interleukin-1 converting enzyme (ICE). This calcium ion-activated serine protease, also called caspase-1 (Sect. 13.4.1), is secreted by fibroblasts and macrophages. The active form of IL-1β is therefore one third smaller than IL-1α. Other proteases secreted by the successor microbiota, for example, T. denticola in the climax microbial complex (Fig. 13.3), also activate IL-1β. Keratinocytes, including junctional epithelial basal cells exposed to PAMPs, secrete IL-1β along with IL-1α, whereas fibroblasts and macrophages secrete IL-1β and ICE.
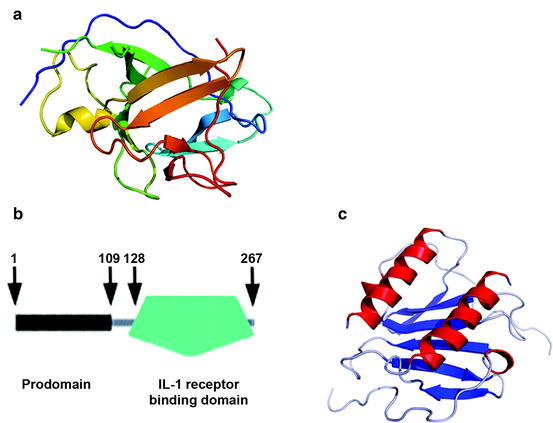

Fig. 13.6
Structures of IL-1α and IL-8. (a) Structures of Interleukin-1. Interleukins are a family of intercellular signaling molecules (cytokines) that are ligands for a specific receptor, the IL-1 receptor. IL-1 forms a 12-stranded β-sheet structure, several regions of which, but especially a loop between strands 4 and 5, are implicated in binding to the IL-1 receptor. (b) Domain structure of IL-1. IL-1α is composed of an N-terminal prodomain (residues 1–109), a short unfolded connector region (residues 110–128) and a large C-terminal domain (residues 129–267) containing the β-sheet conformation shown in (a) above. IL-1β is similarly constructed, but two residues shorter than IL-1α. IL-1α is active without cleavage, whereas the prodomain of IL-1β, which ends at residue 103, prevents receptor-binding unless it is cleaved in the connector domain (residues 104 – 118) C-terminal to aspartate residue 116 (see text). The large fragment contains a homologous β-sheet domain (residues 119–265). (c) Interleukin-8 (IL-8). IL-8 is not of the interleukin family. It is one of a family of 13 CXC chemokines, small, positively charged proteins that attract and activate leukocytes (see text). The many chemokines in the body are divided into CXC and CC subfamilies, based on the arrangement of the first two of four conserved cysteine residues; the two cysteines are separated by a single amino acid in CXC chemokines but lie next to each other in CC chemokines. Chemokine structures all have a double α-helix that interacts with the receptor following heparin binding (a: Figure is public domain; http://en.wikipedia.org/wiki/Image:2ILA.png; b: Figure is modified from an EMBO SMART diagram of IL-1 at http://smart.embl.de/smart/show_motifs.pl?ID=P01583; c
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


