Oral mucositis is a significant toxicity of systemic chemotherapy and of radiation therapy to the head and neck region. The morbidity of oral mucositis can include pain, nutritional compromise, impact on quality of life, alteration in cancer therapy, risk for infection, and economic costs. Management includes general symptomatic support and targeted therapeutic interventions for the prevention or treatment of oral mucositis. Evidence-based clinical practice guidelines are available to guide clinicians in the selection of effective management strategies.
Key points
- •
Oral mucositis is a significant toxicity of systemic chemotherapy and of radiation therapy to the head and neck region.
- •
The morbidity of oral mucositis can include pain, nutritional compromise, impact on quality of life, alteration in cancer therapy, risk for infection, and economic costs.
- •
Management includes general symptomatic support and targeted therapeutic interventions for the prevention or treatment of oral mucositis.
- •
Evidence-based clinical practice guidelines are available to guide clinicians in the selection of effective management strategies.
Introduction, epidemiology, and pathogenesis
An estimated 1.6 million people receive cancer therapy in the United States each year and worldwide numbers are much higher. Although advancements in cancer therapy improved survival rates for many tumor types, these treatments also cause several side-effects, including some in the oral cavity. One of the more significant oral complications of cancer therapy is oral mucositis, which refers to inflamed erosive or ulcerative lesions of the oral mucosa. Oral mucositis can result from systemic chemotherapy, from radiation therapy (RT) to the oral mucosa, or a combination thereof. It affects approximately 20% to 40% of patients receiving conventional chemotherapy regimens for solid tumors, depending on the dose and cytotoxicity of the drug. In patients receiving very high doses of chemotherapy before a hematopoietic stem cell transplant (HSCT), oral mucositis is seen in about 80%. Almost all patients receiving therapeutic radiation for head and neck (H&N) cancer will develop oral mucositis. Collectively in the United States, it is estimated that about 400,000 patients suffer from oral mucositis each year.
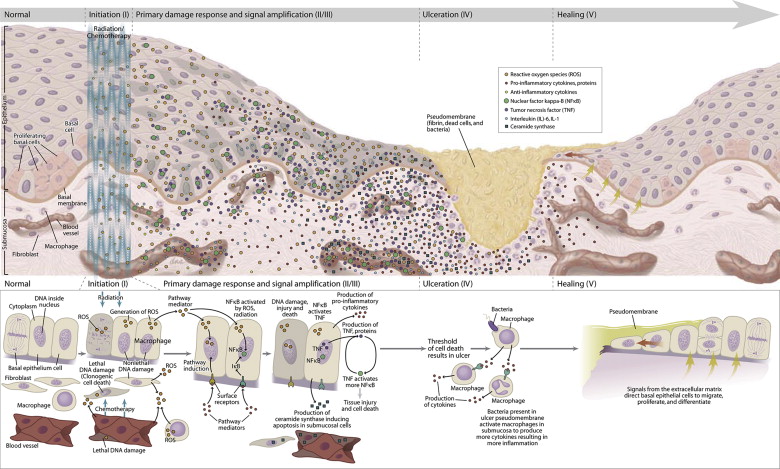
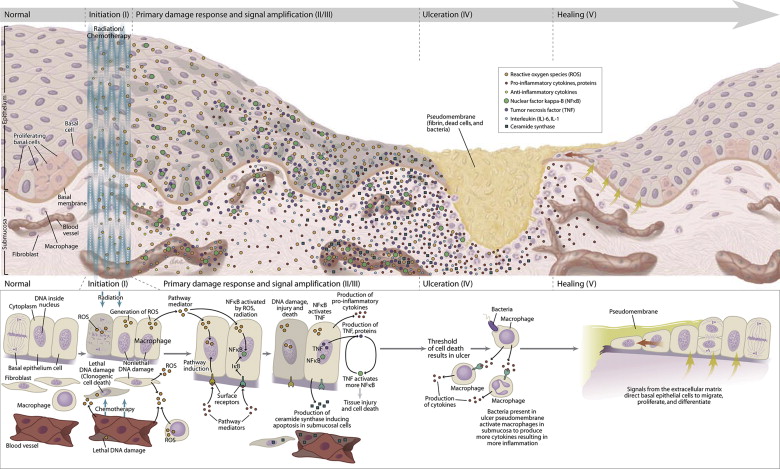
Historically, mucositis was thought to result only from damage to basal epithelial cells due to chemotherapy or RT. It is now understood that the pathogenesis is much more complex and involves the generation of damaging reactive oxygen species, activation of transcription factors such as nuclear factor-κB and inflammatory pathways such as the cyclooxygenase pathway, and the upregulation of proinflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-1β. The various factors involved have been integrated into a five-step pathogenesis model ( Fig. 1 ).
Introduction, epidemiology, and pathogenesis
An estimated 1.6 million people receive cancer therapy in the United States each year and worldwide numbers are much higher. Although advancements in cancer therapy improved survival rates for many tumor types, these treatments also cause several side-effects, including some in the oral cavity. One of the more significant oral complications of cancer therapy is oral mucositis, which refers to inflamed erosive or ulcerative lesions of the oral mucosa. Oral mucositis can result from systemic chemotherapy, from radiation therapy (RT) to the oral mucosa, or a combination thereof. It affects approximately 20% to 40% of patients receiving conventional chemotherapy regimens for solid tumors, depending on the dose and cytotoxicity of the drug. In patients receiving very high doses of chemotherapy before a hematopoietic stem cell transplant (HSCT), oral mucositis is seen in about 80%. Almost all patients receiving therapeutic radiation for head and neck (H&N) cancer will develop oral mucositis. Collectively in the United States, it is estimated that about 400,000 patients suffer from oral mucositis each year.
Historically, mucositis was thought to result only from damage to basal epithelial cells due to chemotherapy or RT. It is now understood that the pathogenesis is much more complex and involves the generation of damaging reactive oxygen species, activation of transcription factors such as nuclear factor-κB and inflammatory pathways such as the cyclooxygenase pathway, and the upregulation of proinflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-1β. The various factors involved have been integrated into a five-step pathogenesis model ( Fig. 1 ).
Morbidity of oral mucositis
Pain
The primary morbidity of oral mucositis is the intense pain usually associated with ulcerative lesions. Most patients with ulcerative mucositis need systemic opioids for pain management. In one study, a 1-point increase in peak mucositis scores in patients with HSCT was associated with 2.6 additional days of injectable narcotic therapy. Systemic opioids have several side-effects, including risk of dependence, altered mental state, and constipation.
Nutritional Compromise
Because of the pain, patients with severe oral mucositis may be unable to continue eating by mouth. Therefore, they are fed intravenously via total parenteral nutrition (TPN) or through a gastrostomy tube. A 1-point increase in peak mucositis scores in HSCT patients was associated with 2.7 additional days of TPN. Patients who have H&N cancer with oral mucositis are significantly more likely to have weight loss of 5% or more. Impaired nutrition affects healing and resistance to infection, and leads to a general failure to thrive.
Quality of Life
The pain and nutritional compromise adversely affects quality of life. This is especially prominent in patients receiving H&N RT and in patients receiving high-dose chemotherapy for HSCT. Patients undergoing HSCT report oral mucositis as the most debilitating complication of transplantation.
Impact on Cancer Therapy
Severe mucositis can necessitate undesirable dose reductions or interruptions in cancer therapy. In patients receiving chemotherapy for solid tumors or lymphoma, a reduction in the next dose of chemotherapy was twice as common after cycles with mucositis than after cycles without mucositis. Eleven percent of patients receiving RT for H&N cancer had unplanned breaks in RT because of severe mucositis. Such modifications in cancer therapy can negatively affect cancer prognosis.
Infection
Ulcerative oral mucositis is colonized by oral microflora and is sometimes complicated by local infection such as herpes simplex virus (HSV) infection and candidiasis. In patients who are immunosuppressed due to chemotherapy, these ulcerative lesions can provide a route for systemic sepsis, which is potentially life threatening. For example, in patients receiving conventional chemotherapy for solid tumors or lymphoma, the rate of infection during cycles with mucositis was more than twice that of cycles without mucositis and was proportional to its severity. Infection-related deaths were more common during cycles with mucositis. In patients receiving high-dose chemotherapy for HSCT, moderate to severe oral mucositis has been correlated with systemic infection and transplant-related mortality. Increased severity of oral mucositis has been associated with increased incidence of significant infection, number of days with fever, and a 3.9-fold increase in 100-day mortality risk.
Impact on Oral Health
Patients with painful oral mucositis may have difficulty carrying out routine oral hygiene measures such as brushing and flossing. In addition, transient or permanent hyposalivation is a frequent finding in patients receiving cancer therapy. In combination, these factors can increase the risk of dental caries and periodontal disease. Furthermore, as mentioned above, lesions of oral mucositis can be secondarily infected with oral candidiasis or HSV, in the setting of possible immunosuppression. Thus, oral mucositis has a significant negative impact on oral and dental health.
Economic Impact
Oral mucositis results in increased costs associated with pain management, TPN or gastrostomy tubes with liquid diet supplements, secondary infections, and hospitalizations. In patients receiving H&N RT, oral mucositis was associated with an increase in costs ranging from $1700 to $6000 per patient, depending on severity of mucositis. In patients receiving conventional dose chemotherapy for solid tumors, average duration of hospitalization was significantly longer for cycles with mucositis and cost of hospitalization was 60% higher in cycles with oral mucositis. In patients receiving high-dose chemotherapy for HSCT, increased severity of oral mucositis was associated with increased time in hospital, and increased total inpatient charges. A single-point increase in oral mucositis severity was associated with 2.6 additional days in hospital and more than $25,000 in additional hospital charges.
Clinical findings
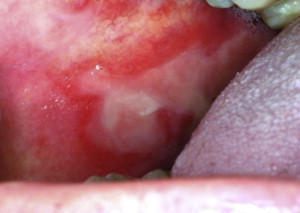
Diagnosis of oral mucositis is usually made clinically, based on clinical appearance and a history of cytotoxic cancer therapy within the expected timeframe. It initially presents as erythema of the oral mucosa, which often progresses to erosion and frank ulceration. The ulcerations are typically covered by a pseudomembrane ( Fig. 2 ). The nonkeratinized mucosa is more often affected. Chemotherapy-induced oral mucositis presents 7 to 14 days after initiation of chemotherapy and typically heals within a few weeks after end of chemotherapy. In patients receiving the typical 6 to 7 week regimen of RT for H&N cancer, onset of oral mucositis occurs by the second or third week of RT, and the severity worsens with increasing dose of RT. The areas affected are defined by the field of radiation ( Fig. 3 ). The duration typically extends for several weeks after the end of RT, depending on the size of the lesions. In cases in which the clinical appearance or duration is not consistent with mucositis, secondary infection or an alternative diagnosis should be considered. The most common clinically relevant secondary infections of oral mucositis lesions involve Candida species fungi and HSV. These are more likely to be seen in patients who are immunosuppressed due to chemotherapy or in patients with hyposalivation due to RT.