Osteonecrosis of the jaw is a major public health concern throughout the world. Use of radiotherapy for head and neck cancer and bone antiresorptives and antiangiogenic agents have increased its incidence. Medication-related osteonecrosis of the jaw is more common relative to other types of osteonecrosis. Osteoradionecrosis occurs despite better treatment planning and shielding to minimize collateral damage to bone. Other related necrotic lesions are secondary to usage of recreational drugs and steroids. This article provides comprehensive information about these different types of bone necrosis; provides the readers with radiographic diagnostic criteria and updates on current theories on pathophysiology of osteonecrosis.
Key points
- •
The role of radiographic imaging in diagnosis of osteonecrotic lesions cannot be overemphasized.
- •
Treatment options for jaw osteonecrosis include the use of local and systemic antibiotics, pain medications, debriding, sequestrectomy, hyperbaric oxygen treatment, and use of the antioxidants, tocopherol and Pentoxifylline. Surgical resection is usually a last resort, when all other forms of therapy fail.
- •
The effects on the quality of life in patients with jaw osteonecrosis, makes it an important area of research, especially to researchers interested in bone and tissue engineering.
Introduction
Bone is a unique connective tissue because it is functionally dynamic, consisting of different cells that continuously interact together. Unlike other connective tissues within the body, bone is physiologically mineralized. There is also an abundance of osteoprogenitor cells that reside within the bone microenvironment that can be activated to form different cell types. The ability of bone to constantly remodel plays a vital role in the maintenance of mineral homeostasis, as old bone is removed by the activities of osteoclasts and new bone matrix is deposited by osteoblasts. Essentially, external and internal insults from radiation, drugs, or other chemical insults can induce a pathologic process that disrupts the bone microenvironment, turnover, and homeostasis. The outcome is dysregulation of the bone healing process that can potentially lead to loss of bone tissue, as in osteonecrosis.
Osteonecrosis is characterized by tissue dehiscence, chronic bone devitalization, hypocellularity, and osteolysis. The term osteonecrosis is often used interchangeably with ischemic necrosis, avascular necrosis, or aseptic necrosis, but there are different types of osteonecrosis. Depending on the etiologic agent, osteonecrosis can occur in any bone including the orofacial, appendicular, and axial bones. Osteonecrosis may or may not be associated with exposed bone with delayed healing. Specifically, the femoral head and mandible are highly susceptible to osteonecrosis. In the orofacial region, jaw osteonecrosis can lead to significant loss of bone tissue, tooth loss, and facial disfigurement. The unfortunate outcomes are significant morbidity, debility, and diminished quality of life. The high susceptibility of the femoral bone to osteonecrosis is associated with a variety of factors that include alcohol abuse and steroid therapy. However, jaw osteonecrosis is much more associated with complications of radiation therapy, and long-term therapy with bone antiresorptives used to control skeletal events of cancer metastasis and osteoporosis. In a randomized controlled study that assessed 792 cases of osteonecrosis in general, 76% of the cases occurred in the hip, and 4.4% occurred in the jaw mainly as a result of bisphosphonate therapies. The remaining cases were associated with the wrist, knee, foot, or ankle. Several pathophysiologic theories have been proposed for osteonecrosis based on correlations of clinical signs with histologic and radiologic analyses. Although many of these theories have not been conclusively established, radiographic imaging has played a major role in the diagnosis, management, and follow-up assessment of osteonecrosis. Even more importantly is the increasing use of the combination of functional imaging with planar images to fully understand the metabolic changes that lead to osteonecrosis.
Introduction
Bone is a unique connective tissue because it is functionally dynamic, consisting of different cells that continuously interact together. Unlike other connective tissues within the body, bone is physiologically mineralized. There is also an abundance of osteoprogenitor cells that reside within the bone microenvironment that can be activated to form different cell types. The ability of bone to constantly remodel plays a vital role in the maintenance of mineral homeostasis, as old bone is removed by the activities of osteoclasts and new bone matrix is deposited by osteoblasts. Essentially, external and internal insults from radiation, drugs, or other chemical insults can induce a pathologic process that disrupts the bone microenvironment, turnover, and homeostasis. The outcome is dysregulation of the bone healing process that can potentially lead to loss of bone tissue, as in osteonecrosis.
Osteonecrosis is characterized by tissue dehiscence, chronic bone devitalization, hypocellularity, and osteolysis. The term osteonecrosis is often used interchangeably with ischemic necrosis, avascular necrosis, or aseptic necrosis, but there are different types of osteonecrosis. Depending on the etiologic agent, osteonecrosis can occur in any bone including the orofacial, appendicular, and axial bones. Osteonecrosis may or may not be associated with exposed bone with delayed healing. Specifically, the femoral head and mandible are highly susceptible to osteonecrosis. In the orofacial region, jaw osteonecrosis can lead to significant loss of bone tissue, tooth loss, and facial disfigurement. The unfortunate outcomes are significant morbidity, debility, and diminished quality of life. The high susceptibility of the femoral bone to osteonecrosis is associated with a variety of factors that include alcohol abuse and steroid therapy. However, jaw osteonecrosis is much more associated with complications of radiation therapy, and long-term therapy with bone antiresorptives used to control skeletal events of cancer metastasis and osteoporosis. In a randomized controlled study that assessed 792 cases of osteonecrosis in general, 76% of the cases occurred in the hip, and 4.4% occurred in the jaw mainly as a result of bisphosphonate therapies. The remaining cases were associated with the wrist, knee, foot, or ankle. Several pathophysiologic theories have been proposed for osteonecrosis based on correlations of clinical signs with histologic and radiologic analyses. Although many of these theories have not been conclusively established, radiographic imaging has played a major role in the diagnosis, management, and follow-up assessment of osteonecrosis. Even more importantly is the increasing use of the combination of functional imaging with planar images to fully understand the metabolic changes that lead to osteonecrosis.
Types of osteonecrosis
Osteoradionecrosis
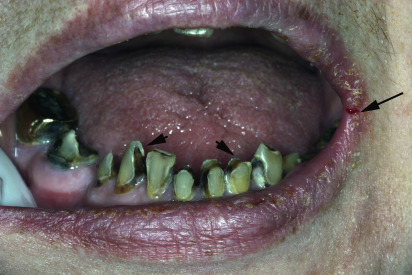
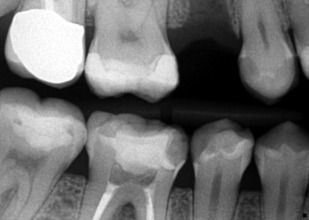
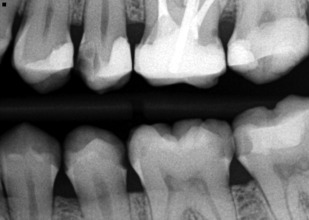
Osteoradionecrosis (ORN) of the jaw is defined as nonhealing bony exposure and necrosis that starts with a breach in the oral mucosa, and persists for at least 3 months, in a patient who has undergone previous radiation therapy. The necrosis, however, must be evidently different from a recurrent, vestigial, or metastatic tumor. This definition, however, does not include cases of ORN in which the oral mucosa is intact, but osteonecrotic changes can be observed by diagnostic imaging. ORN is a chronic condition that can last for months or even years after the initial radiation therapy. The incidence of ORN can range from 2.6% to 22.0% and it develops when the radiation dose exceeds 50 Gy. Specifically, radiation doses between 50 and 70 Gy have been implicated in the etiology of ORN. Within the orofacial complex, the mandible is commonly affected because the mandible is usually in the line of radiation delivery and it is believed that the mandible is less vascularized than the maxilla. Radiation also affects teeth secondarily, due to pronounced xerostomia noted in patients receiving radiation therapy ( Fig. 1 ). The extensive carious lesions can be readily noted on bitewing radiographs, as demonstrated in Figs. 2 and 3 .
Pathogenesis
Osteoradionecrosis was first described in 1926. It was not until 1970 that a triad of radiation, trauma, and infection was proposed as the mechanistic process in ORN. However, this theory was later replaced in 1983 by another proposal that radiation causes development of hypoxic-hypocellular-hypovascular tissue (3H theory), when it was reported that microorganisms do not play any causative but rather a contaminant role in ORN. It was also reported that trauma mainly creates a portal of entry for microorganisms to invade the radiation-suppressed bone. The 3H theory of ORN takes into account that several tissues from exterior to interior are damaged by radiation, ranging from the skin or mucosa to periosteum, bone, and finally endothelium within the bone marrow compartment. So the combination of tissue fibrosis, vascular and cellular damage induces a hypoxic environment within the radiated tissue. The 3H theory was quickly followed by development of hyperbaric oxygen (HBO) therapy protocols to prevent and treat ORN, but this had only modest effectiveness, and HBO therapy is limited because it is contraindicated in patients with metastatic cancer.
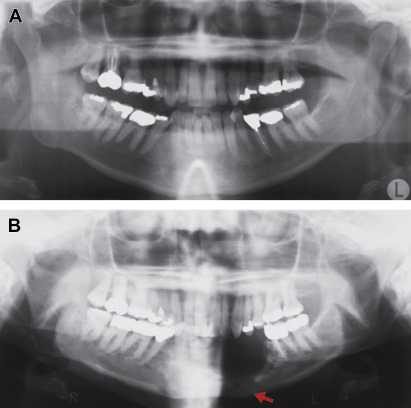
The radiation-induced fibroatrophic process is another mechanism associated with radiation damage more commonly to the superficial structures. This is associated with the activity of reactive oxygen species that cause damage to fibroblasts, endothelium, and bone cells, eventually causing tissue and bone necrosis ( Fig. 4 ). However, the direct application of this theory to deep-seated radiation damage within the bone is yet to be conclusively clarified. Another proposed pathophysiologic hypothesis is that ORN can be precipitated by a combination of dysregulated turnover, osteoclast depletion, local tissue injury, and infection. As all these theories do not conclusively define the pathophysiological process of ORN, more research is still needed to further our understanding of ORN pathogenesis.
Risk factors and classification
Several risk factors predispose to ORN; these include poor oral health, smoking, alcohol abuse, and most importantly, type and dose of radiation. Brachytherapy and radiation doses greater than 50 Gy have been associated with higher incidence of ORN. Additionally, any surgical manipulations of the irradiated area, including dental extractions, pose a significant risk. Several different classifications of ORN have been proposed, but one recently proposed combines clinical description, presence or absence of symptoms, and the treatment option for each of the different stages of ORN ( Table 1 ).
| Stage | Length of Affected Bone/Associated Structures (Damaged/Exposed) | Presence/Absence of Symptoms | Treatment |
|---|---|---|---|
| 1 | <2.5 cm | Asymptomatic. | Medication treatment only. |
| 2 | >2.5 cm | Asymptomatic. Includes pathologic fracture and/or involvement of inferior dental nerve. |
Medication treatment only; except for presence of dental sepsis and loose/necrotic bone. |
| 3 | >2.5 cm | Symptomatic. No other features of bone necrosis. However, symptoms persist despite medication treatment. |
Debridement of loose/necrotic bone. Local pedicle flap. |
| 4 | >2.5 cm | Symptomatic. Pathologic fracture. Involvement of inferior alveolar nerve and/or orocutaneous fistula. |
Reconstruction with free flap, if patient’s overall health allows. |
Clinical presentation
Patients with ORN usually present with pain that is typically neuropathic in nature. Also swelling may be accompanied by fever depending on the extent of the inflammatory process. Follow-up clinical evaluation may reveal tissue breakdown and bone necrosis that may be associated with paresthesia/anesthesia. If untreated, ORN especially in the mandible may result in pathologic fracture. Definitive diagnosis of ORN is a combination of clinical, radiologic, and histologic evaluations.
Medication-Related Osteonecrosis of the Jaws
Medication-related osteonecrosis of the jaw (MRONJ) is a more recent class of jaw osteonecrosis first described in 2003 in patients taking nitrogen-containing bisphosphonates (nBPs). It is defined as exposed bone in the intraoral cavity persisting for 8 weeks or more, in patients who have previously undergone, or are currently undergoing treatment with antiresorptives and/or antiangiogenic agents and with no previous history of radiation therapy to the jaw. This excludes primary or metastatic cancer within the jaw region. However, this definition does not take into account the nonexposed bone variant of the disease process, which makes up about a third of all cases of MRONJ cases.
The nBPs, especially the intravenous nBPs, such as zoledronic acid, were the first group of drugs initially associated with MRONJ. The high efficacy of intravenous nBPs, such as zoledronic acid and pamidronate, to control altered bone remodeling make them highly favored for the treatment of skeletal events of cancer metastasis, Paget disease, osteogenesis imperfecta, and hyperparathyroid jaw tumors, so it is understandable that nBPs were the first to be associated with osteonecrosis exclusive to the jaws. Other medications also have been implicated in MRONJ; these include another antiresorptive drug, denosumab, which acts as an inhibitor of receptor activator for NFκB ligand (RANKL), and antiangiogenic drugs like bevacizumab, an inhibitor of vascular endothelial growth factor, and sunitinib, a tyrosine kinase inhibitor.
Because of the vast array of medications associated with osteonecrosis of the jaw (ONJ), the nomenclature for this disorder has evolved over the years from ONJ, bisphosphonate osteonecrosis (BON), bisphosphonate-related osteonecrosis of the jaw (BRONJ), and anti-resorptive osteonecrosis of the jaw (ARONJ) to the more recent MRONJ. The incidence of MRONJ in patients taking intravenous nBPs ranges from 0% to 27.5%, and denosumab 1.7%. The relative risks of MRONJ occurring in patients taking intravenous nBPs, denosumab, or bevacizumab are 0.7% to 6.7%, 0.7% to 1.9%, and 0.2% respectively. MRONJ affects the mandible and maxilla at a ratio of 2:1, because the mandible is partly associated with a single vascular supply from the inferior alveolar artery compared with the superior, inferior, and middle arteries in the maxilla. Severe cases of MRONJ in the mandible can lead to pathologic fracture, whereas in the maxilla it can result in oroantral fistulation.
Pathogenesis
The pathophysiology of MRONJ is still unclear, but different investigators have proposed several theories. These include a decrease in bone turnover, presence of infection (especially Actinomyces species), inhibition of angiogenesis, and a dysregulation or dysfunction of innate and acquired immunity. The infection theory is based on the premise that a “complex biofilm” is present on the surface of exposed necrotic bone. Definitive elucidation of MRONJ pathogenesis is hampered because the offending drugs have different mechanisms of action ( Table 2 ) and nonoral bones are spared by MRONJ. The role of bone mesenchymal stem cells (MSCs) also cannot be overlooked, considering that jaw MSCs are phenotypically and functionally different from those of axial and appendicular bones and they are disproportionately more sensitive to both zoledronic acid and pamidronate, both of which are strongly associated with MRONJ.



