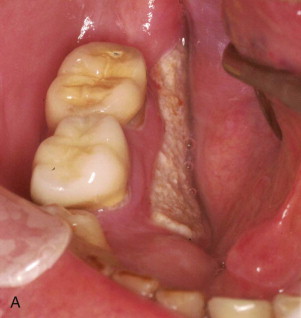
Though a bisphosphonate, etidronate (Didronel) was introduced in the 1980s mostly for the treatment of Paget disease and bony ankylosis; it was not until the early 2000s that cases of exposed bone in the jaws, now known as bisphosphonate-induced osteonecrosis of the jaws (BIONJ) ( Fig. 65-1 ), became evident. This was largely the result of the introduction of the vastly more potent intravenous bisphosphonates pamidronate (Aredia) and zoledronate (Zometa) for the treatment of hypercalcemia of malignancy and stabilization of metastatic malignancies in bone, as well as the oral bisphosphonate alendronate (Fosamax) for the treatment of osteoporosis. It was available in 1995 but did not become popular until 1999. The increased potency was due to pharmacologic restructuring of the basic bisphosphonate molecule in which nitrogen-containing side chains were added to the backbone carbon.

Today, seven bisphosphonates are currently available in the United States: etidronate (Didronel), tiludronate (Skelid), risedronate (Actonel), alendronate (Fosamax), ibandronate (Boniva), pamidronate (Aredia), and zoledronate (Zometa, 4 mg intravenously once monthly, and Reclast, 5 mg intravenously once every 1 to 2 years). All except etidronate and tiludronate have a nitrogen-containing side chain, which makes all of them much more potent than either etidronate or tiludronate. Alendronate and pamidronate are 5000 times more potent than etidronate, and zoledronate is 10,000 times more potent than etidronate. Only bisphosphonates with a nitrogen-containing side chain cause BIONJ.
Bisphosphonate Chemistry and Pharmacology
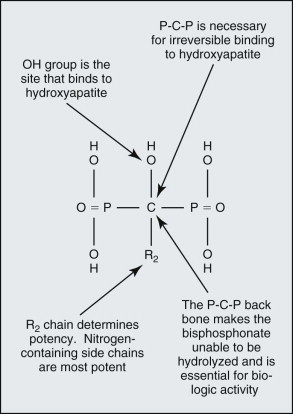
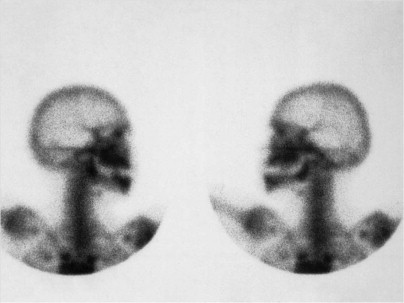
The basic bisphosphonate molecule is shown in Figure 65-2 . The phosphate components impart an affinity for bone from the systemic circulation in the same way that diagnostic technetium Tc 99m methylene diphosphonate bone scan radionuclides collect in more active areas of bone turnover ( Fig. 65-3 ). The backbone carbon provides strong binding to hydroxyapatite in bone, which is made even more irreversible with the addition of an OH group on the backbone carbon. The binding site on the backbone carbon atom opposite this OH group is the site for the nitrogen-containing side chains that confer potency. The result is a bisphosphonate molecule that is irreversibly bound to bone and cannot be metabolized by the human body. Its half-life in bone has been found to be longer than 11 years. The only way that it can be removed from bone is by osteoclastic resorption, which in turn causes apoptosis (cell death) of the osteoclast.
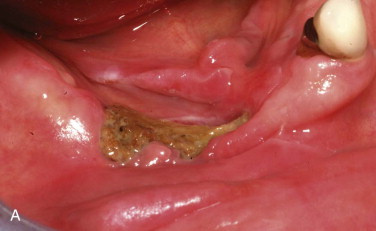
Intravenous administration of a bisphosphonate results in renal clearance of 30% to 40% of the drug. The remaining 60% to 70% becomes bound to bone with a half-life of 11 years. Therefore, some renal toxicity, as well as BIONJ, has been reported with intravenous bisphosphonates. Oral administration of a bisphosphonate results in only 0.64% of the dose actually being absorbed into the systemic circulation, 30% to 40% of which is cleared in the kidneys. The result is that renal toxicity is not seen with oral bisphosphonates, but frequent severe esophagitis and even rare esophageal cancers have been reported with oral bisphosphonates, presumably because of residual non-absorbed bisphosphonate in contact with the esophageal mucosa. Related to oral bisphosphonates, intravenous bisphosphonates have 140 times the bone bioavailability. The result is that clinical BIONJ caused by intravenous bisphosphonates appears much sooner, is generally more severe, and is less responsive to discontinuation of use of the drug ( Fig. 65-4 ).
Mechanism of Action and Pathology
The Bone-Remodeling Cycle
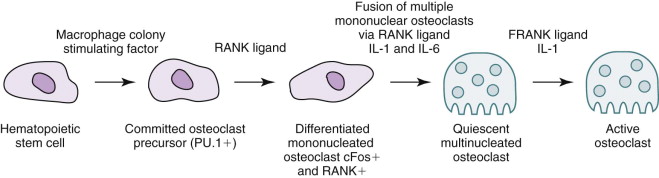
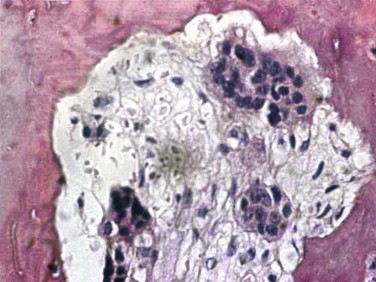
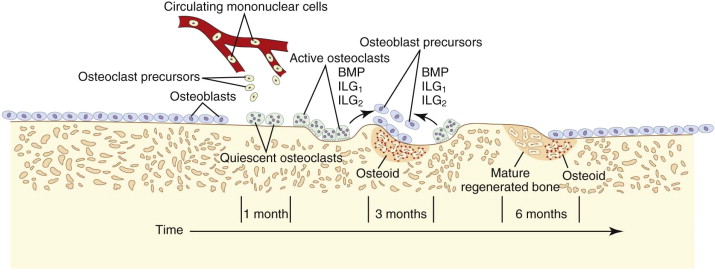
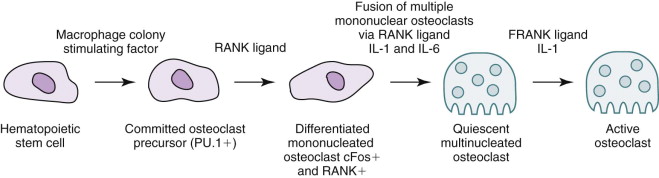
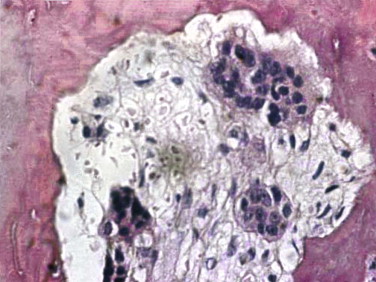
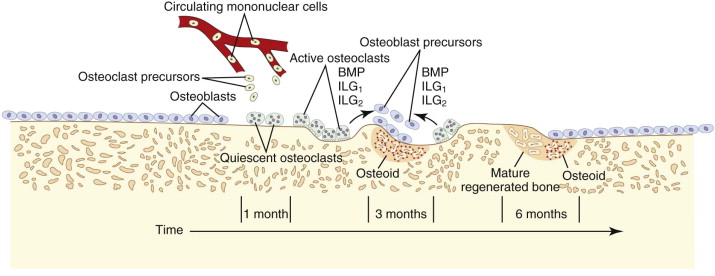
The target of both the therapeutic and adverse effects of bisphosphonates is the osteoclast. Osteoclasts are multinucleated cells that arise from mononuclear bone marrow precursors in a series of maturation steps involving macrophage colony-stimulating factor, receptor activator of nuclear factor κB ligand (RANKL), interleukin-1, and interleukin-6 ( Fig. 65-5 ) into a mature but quiescent osteoclast with a life span of only 14 days. In the normal state, RANKL activates quiescent osteoclasts to actively resorb bone. However, normal bone secretes osteoprotegerin, which competitively inhibits RANKL. Therefore, young healthy bone is not resorbed and the overall skeleton remains stable. However, osteocytes that live for about 180 days become old, and their secretion of osteoprotegerin drops, which allows RANKL to cause resorption of only this old or dead bone ( Fig. 65-6 ). Therefore, evolution has created a mechanism of identifying and eliminating weak and structurally unsound older bone. It has also created a mechanism to replace it with new, more elastic and structurally more supportive bone. That is, during the resorption process, bone morphogenetic protein (BMP) and insulin-like growth factors I and II (IGF-I and IGF-II), which were placed in the bone matrix by osteoblasts at the time of bone formation, are released. These growth and differentiation factors stimulate new bone formation to replace the resorbed bone, thus renewing bone and maintaining skeletal mass and strength ( Fig. 65-7 ).
Strategies in Bisphosphonate Therapeutics
Most cancers do not resorb bone by themselves. Instead, they secrete RANKL and RANKL-like substances that locally activate osteoclasts to resorb bone for them. The cancer merely proliferates into the resorbed space. Hence, metastatic cancer deposits accumulate in bone and weaken it to the point of a pathologic fracture, which causes pain and morbidity. In many cancers, excessive bone resorption results in hypercalcemia, whereas in others (notably small cell carcinoma of the lung), a parathyroid hormone–like peptide is secreted that induces osteoclastic bone resorption and subsequent hypercalcemia. By literally killing off osteoclasts with bisphosphonates, the cancer cannot resorb bone and calcium levels are returned to normal, thereby sparing patients the pain and disability of pathologic fractures and the symptoms of hypercalcemia, such as mental confusion and constipation.
In the treatment of osteoporosis with oral bisphosphonates, their interruption of bone renewal results in greater density of bone by the accumulation of older and more mineralized bone. This is measured by dual-energy x-ray absorptiometry (DEXA). However, although DEXA measures increased bone mineral density, which adds bone strength and resistance to fracturing up to a point, repeated use of an oral bisphosphonate builds up old and more brittle bone, which may actually fracture more easily. Long-bone fractures caused by the use of alendronate for longer than 6 years has already been reported in the literature, and prospective randomized double-blind studies have shown that the therapeutic effects of alendronate cease after 5 years of use. Coupled with the knowledge that the incidence of BIONJ increases and clinical severity worsens after 3 years, the editor of The Journal of the American Medical Association stated in the journal that “alendronate—five years of a good thing is enough.”
Pathophysiology of Bisphosphonate-Induced Osteonecrosis of the Jaws
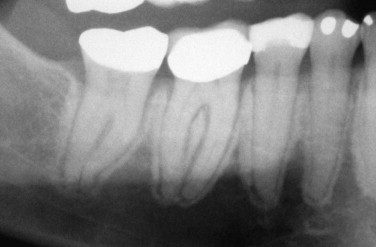
BIONJ always begins in the alveolar bone or in the surface bone of mandibular or maxillary tori. This is due to the more rapid turnover rate of these bony areas than that found in any other part of the adult skeleton. It is sometimes seen as sclerosis of the lamina dura and widening of the periodontal ligament space because this bone remodels in response to occlusal forces ( Fig. 65-8 ). Since the molar areas have a wider occlusal table and compressive forces are greater on the molars, there is a higher incidence of BIONJ in the molar regions. Even in edentulous patients the target area remains alveolar bone because of compressive forces from denture wearing on the alveolar crest or around dental implants that are loaded for denture function.
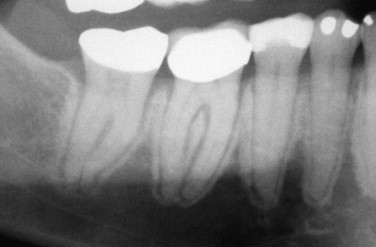
Essentially, bisphosphonates prevent the normal renewal of bone in the lamina dura and the edentulous alveolar crest, which results in retention of old bone that dies off and is not replaced. Such necrotic bone fails to support the blood supply of the overlying mucosa and therefore results in loss of mucosa and bone exposure. The exposed bone in turn may be colonized by microorganisms and give rise to pain from an inflammatory reaction around the colonized area on the bone surface and secondary infection of the surrounding soft tissues. In some cases the microorganism invades into the bone and produces secondary osteomyelitis. The exposed bone by itself is not painful; it is necrotic and absent of blood vessels and nerves. It is only when secondary infection of the soft tissues or, more rarely, the bone occurs that the condition becomes painful.
Mechanism of Action and Pathology
The Bone-Remodeling Cycle
The target of both the therapeutic and adverse effects of bisphosphonates is the osteoclast. Osteoclasts are multinucleated cells that arise from mononuclear bone marrow precursors in a series of maturation steps involving macrophage colony-stimulating factor, receptor activator of nuclear factor κB ligand (RANKL), interleukin-1, and interleukin-6 ( Fig. 65-5 ) into a mature but quiescent osteoclast with a life span of only 14 days. In the normal state, RANKL activates quiescent osteoclasts to actively resorb bone. However, normal bone secretes osteoprotegerin, which competitively inhibits RANKL. Therefore, young healthy bone is not resorbed and the overall skeleton remains stable. However, osteocytes that live for about 180 days become old, and their secretion of osteoprotegerin drops, which allows RANKL to cause resorption of only this old or dead bone ( Fig. 65-6 ). Therefore, evolution has created a mechanism of identifying and eliminating weak and structurally unsound older bone. It has also created a mechanism to replace it with new, more elastic and structurally more supportive bone. That is, during the resorption process, bone morphogenetic protein (BMP) and insulin-like growth factors I and II (IGF-I and IGF-II), which were placed in the bone matrix by osteoblasts at the time of bone formation, are released. These growth and differentiation factors stimulate new bone formation to replace the resorbed bone, thus renewing bone and maintaining skeletal mass and strength ( Fig. 65-7 ).
Strategies in Bisphosphonate Therapeutics
Most cancers do not resorb bone by themselves. Instead, they secrete RANKL and RANKL-like substances that locally activate osteoclasts to resorb bone for them. The cancer merely proliferates into the resorbed space. Hence, metastatic cancer deposits accumulate in bone and weaken it to the point of a pathologic fracture, which causes pain and morbidity. In many cancers, excessive bone resorption results in hypercalcemia, whereas in others (notably small cell carcinoma of the lung), a parathyroid hormone–like peptide is secreted that induces osteoclastic bone resorption and subsequent hypercalcemia. By literally killing off osteoclasts with bisphosphonates, the cancer cannot resorb bone and calcium levels are returned to normal, thereby sparing patients the pain and disability of pathologic fractures and the symptoms of hypercalcemia, such as mental confusion and constipation.
In the treatment of osteoporosis with oral bisphosphonates, their interruption of bone renewal results in greater density of bone by the accumulation of older and more mineralized bone. This is measured by dual-energy x-ray absorptiometry (DEXA). However, although DEXA measures increased bone mineral density, which adds bone strength and resistance to fracturing up to a point, repeated use of an oral bisphosphonate builds up old and more brittle bone, which may actually fracture more easily. Long-bone fractures caused by the use of alendronate for longer than 6 years has already been reported in the literature, and prospective randomized double-blind studies have shown that the therapeutic effects of alendronate cease after 5 years of use. Coupled with the knowledge that the incidence of BIONJ increases and clinical severity worsens after 3 years, the editor of The Journal of the American Medical Association stated in the journal that “alendronate—five years of a good thing is enough.”
Pathophysiology of Bisphosphonate-Induced Osteonecrosis of the Jaws
BIONJ always begins in the alveolar bone or in the surface bone of mandibular or maxillary tori. This is due to the more rapid turnover rate of these bony areas than that found in any other part of the adult skeleton. It is sometimes seen as sclerosis of the lamina dura and widening of the periodontal ligament space because this bone remodels in response to occlusal forces ( Fig. 65-8 ). Since the molar areas have a wider occlusal table and compressive forces are greater on the molars, there is a higher incidence of BIONJ in the molar regions. Even in edentulous patients the target area remains alveolar bone because of compressive forces from denture wearing on the alveolar crest or around dental implants that are loaded for denture function.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


