Fig. 9.1
Micro-CT images of a maxillary molar demonstrate the root canal complexity (Courtesy Dr. Ronald Ordinala Zapata)
The EndoVac apical negative-pressure irrigation system has three active component parts (Fig. 9.2): the Master Delivery Tip (MDT) (Fig. 9.3), the macrocannula and the microcannula. The MDT accommodates a syringe of irrigant, which is expressed through a 20-gauge needle. There is also a plastic suction hood attached around the 20-gauge needle which is connected to clear plastic tubing which inserts into a multiport adaptor which in turn is inserted into the high-volume suction [13]. As such, the MDT can simultaneously deliver and evacuate any excess irrigant that may flow over from the pulp chamber. The macrocannula is used to draw irrigant by way of suction from the chamber to the coronal and middle segments of the canal, while irrigant is simultaneously delivered to the pulp chamber directed towards an axial wall and never towards a canal orifice. The macrocannula or microcannula is connected via clear plastic tubing to the high-speed suction of the dental unit via the multiport adaptor. The plastic macrocannula (Fig. 9.4) has an external diameter of ISO size of 0.55 mm and an internal diameter of ISO size of 0.35 mm. It is made of blue translucent plastic, has a 0.02 taper and is meant for single use only. It is attached snugly to an autoclavable aluminium hand piece (Fig. 9.5) and is used in an up-and-down pecking motion, while irrigant is simultaneously delivered passively to the pulp chamber in the manner mentioned above. It is used to remove the gross debris and tissue left behind during instrumentation. The microcannula (Fig. 9.6) contains 12 microscopic holes and is capable of evacuating debris to full working length [14]. The size of 0.32-mm-external-diameter stainless-steel microcannula of zero taper has four sets of three laser-cut, laterally positioned offset holes adjacent to its closed end, 100 μ in diameter and spaced 100 μ apart. These holes act as filters to prevent the clogging of the internal lumen of the microcannula which has an internal diameter of ISO size of 0.20 mm. The microcannula is attached to an autoclavable aluminium fingerpiece and is used for irrigation of the apical part of the canal when it is positioned at working length. The microcannula has a closed end and should be taken to the full working length to aspirate irrigants and debris. The microcannula can be used in canals that are enlarged with endodontic files to ISO size 35 with 0.04 taper or larger. A non-tapered preparation can also be considered; in this situation the manufacturer recommends an enlargement of the root canal to 40/0.02.
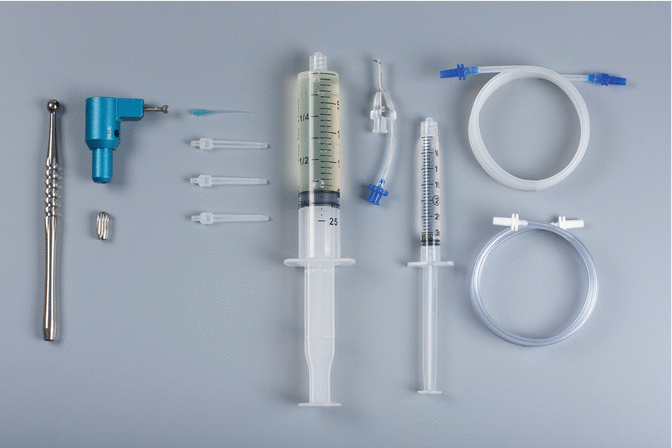
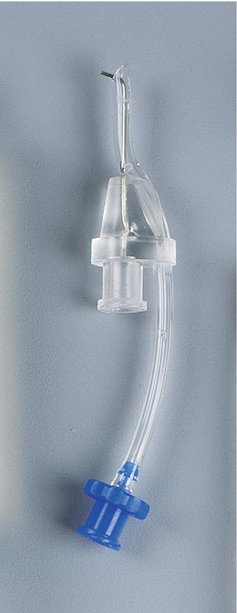


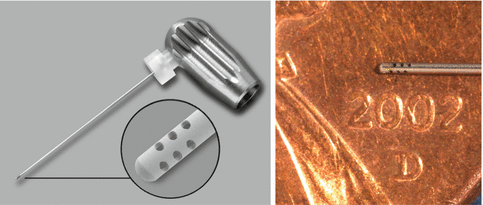

Fig. 9.2
The components of the EndoVac system: the Master Delivery Tip (MDT) accommodates different sizes of syringes filled with irrigant, the macrocannula is attached to the autoclavable aluminium handpiece and the microcannula is attached to an autoclavable aluminium fingerpiece. The macrocannula, the microcannula and the MDT are connected via clear plastic tubing. The tubes are connected to the high-volume suction of the dental chair via the multiport adaptor (Courtesy Dr. John Schoeffel)

Fig. 9.3
Master Delivery Tip (MDT) composed of a 20-gauge needle and luer lock connectors to connect to the syringe and the high-volume suction of the dental chair (Courtesy Kerr Endodontics (SybronEndo). Orange, California)

Fig. 9.4
The macrocannula is made of blue translucent plastic and it is attached to an autoclavable aluminium handpiece (Fig. 9.5) (Courtesy Kerr Endodontics (SybronEndo). Orange, California)

Fig. 9.5
Autoclavable handpiece for the macrocannula (Courtesy Kerr Endodontics (SybronEndo). Orange, California)

Fig. 9.6
The ISO size of 0.32-mm-external-diameter stainless-steel microcannula of zero taper has four sets of three laser-cut, laterally positioned offset holes adjacent to its closed end, 100 μ in diameter and spaced 100 μ apart (Courtesy Dr. John Schoeffel)
During irrigation, the MDT delivers irrigant to the pulp chamber and siphons off the excess irrigant to prevent overflow. Both the macrocannula and microcannula exert negative pressure that pulls fresh irrigant from the chamber, down the canal to the tip of the cannula, into the cannula and out through the suction hose. Thus, a constant flow of fresh irrigant is delivered by negative pressure to working length, allowing the reaction of hydrolysis to continually occur.
Method of Use
Irrigation begins during rotary instrumentation. The MDT delivers fresh irrigant to the access opening when each instrument is changed in the hand piece. Using the MDT is optional during access and the instrumentation phases of root canal treatment. A normal Monoject syringe may be used to replenish the irrigant in the pulp chamber during instrumentation. This removes instrumentation debris and exchanges irrigant deep within the pulp chamber as subsequent files are brought closer and then finally to working length. When using the MDT, always direct the irrigant flow against a chamber wall; never direct the flow of irrigant towards a canal’s orifice as the pressure of irrigant expression has the potential of causing an irrigation accident in straight and wide canals even when the needle is not placed directly in the orifice or canal.
Following complete instrumentation, the macrocannula is used in each canal for 30 s in a short up-and-down pecking motion as close as possible to working length. Continue to deliver copious NaOCl with the MDT while the macrocannula is moving up and down the canal. Observe the macrocannula for continuous flow and that it does not become blocked with debris. If it does, then remove the plastic tubing from the aluminium handle, place a syringe of water tightly at the end and express the water through the handle and macrocannula to dislodge the blockage. This is carefully done over the sink and not over the patient. This step can also be performed with the microcannula should it get blocked. The use of the macrocannula in the final irrigation protocol will remove the gross debris and tissue left behind during instrumentation. If a shortcut is made and this step is not completed for the full 30 s in each canal, then the microcannula used in the next step may get blocked and slow down the irrigation process.
The next step involves three micro cycles. They are called micro cycles because the microcannula is now used at full working length to remove debris from the canal lumen and isthmus areas. Use a ruler to position the rubber stopper that is placed on the microcannula or score the microcannula with an indelible marker (Fig. 9.7). Delicately guide the microcannula to full working length by holding the fingerpiece. The fingerpiece is then released and the tubing is stabilised. The NaOCl is added with the MDT to the pulp chamber for 10 s (Fig. 9.8). After 10 s the irrigant flow is stopped for just a couple of seconds to allow the gas bubbles formed by hydrolysis to be purged from the canal. The NaOCl is added for another 10 s after which the irrigant flow is stopped again to allow the gas bubbles to be purged from the canal. The NaOCl is then added for the third and final time for another 10 s, but at the end of this time period, the microcannula is removed by the fingerpiece as the MDT continues to deliver NaOCl to the pulp chamber as to not allow its removal from the canal just being treated. This allows the canal to be charged (soaked) with fresh NaOCl for 60 s. The first micro cycle allows the organic component of the smear layer to be removed in addition to any fine debris left behind during instrumentation. The second micro cycle using EDTA removes the inorganic component of the smear layer. The microcannula is again delicately guided to full working length. EDTA is added for 10 s, and then the microcannula is removed allowing the canal to be charged for 60 s. As mentioned, this will remove the inorganic component of the smear layer and expose the dentinal tubules in preparation for the third micro cycle. The third micro cycle is the same as the first micro cycle, two purges and a charge for 60 s. Now that the smear layer has been removed from the root canal walls by the first two micro cycles, this third micro cycle will allow the NaOCl to enter the dentinal tubules via osmosis and dissolve the remaining tissue and microbiota [15]. There is no better way to dry the root canals than to delicately guide the microcannula to full working length for just a moment. This is followed by one or two paper points. The canal(s) is now ready for obturation. Refer to Fig. 9.9 for a flow chart illustrating the final irrigation protocol using the EndoVac system.

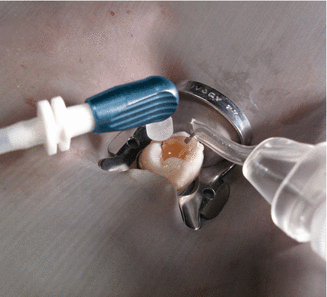
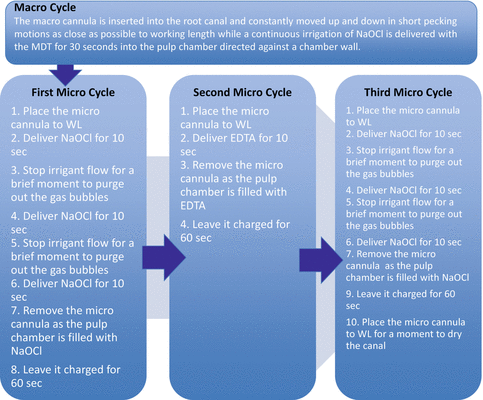

Fig. 9.7
Remove the cap of the microcannula. Use the provided rubber stopper or a marker to indicate working length (Courtesy Kerr Endodontics (SybronEndo). Orange, California)

Fig. 9.8
Once the microcannula is placed at full working length, the clinician may leave it in place and proceed with irrigant delivery via the MDT. Put a slight bend on the microcannula if it won’t stay in the canal on its own (Courtesy Kerr Endodontics (SybronEndo). Orange, California)

Fig. 9.9
Final irrigation protocol using EndoVac system
Debris Removal
Several studies were carried out to evaluate the EndoVac system’s ability to remove debris within the root canal system after instrumentation with rotary files [16–21]. Debridement is a principal objective of root canal treatment and remains a challenge especially in the apical portion of the canal and within the isthmuses and lateral and accessory canals. Debridement is the elimination of organic and inorganic substances as well as microorganisms from the root canal by mechanical and/or chemical means [22]. When compared to traditional syringe and side-vented needle irrigation, the EndoVac system has demonstrated better control to reach the last millimetre of the root canal.
Some in vitro and in vivo studies have demonstrated greater removal of debris from the apical walls and a statistically cleaner result using apical negative-pressure irrigation in closed root canal systems with sealed apices. In an in vivo study of 22 teeth by Siu and Baumgartner, less debris remained at 1 mm from working length using apical negative pressure compared to the use of traditional needle irrigation, while Shin et al. found in an in vitro study of 69 teeth comparing traditional needle irrigation with apical negative pressure that these methods both resulted in clean root canals but that apical negative pressure resulted in less debris remaining at 1.5 and 3.5 mm from working length [18, 23, 24]. When comparing root canal debridement using manual-dynamic agitation (using a well-fitted gutta-percha cone in an up-and-down motion in the canal) or the EndoVac system for final agitation in a closed system and an open system, it was found that the presence of a sealed apical foramen adversely affected debridement efficacy when manual-dynamic agitation was used, but did not adversely affect results when the EndoVac system was used. Apical negative-pressure irrigation is an effective method to overcome the fluid-dynamic challenges inherent in closed root canal systems [25, 26]. The ability of the EndoVac system to significantly clean more debris from a mechanically inaccessible recess of the curved in vitro root canal model may be caused by robust bubble formation during irrigant delivery, creating higher wall shear stresses by a two-phase air–liquid flow phenomenon that is well known in other industrial debridement systems [27]. Less debris remained with the EndoVac system at 1 mm from the working length and in isthmuses [18, 20, 21]. To enhance cleanliness of the root canal system, EndoVac system has the ability to safely deliver irrigant to working length [18] by pulling the irrigant into the canal and removing it by negative pressure [18]. This vacuum action enhances the volume of solution and the circulation of the irrigation solution in the apical end of the root canal. Moreover, the negative pressure avoids air entrapment in the apical third [21] and promotes a regular replenishment of the irrigant apically [21]. A recent study demonstrated that the volume of irrigant delivered apically was significantly higher than the volume delivered by conventional syringe needle irrigation within the same period [18] and resulted in significantly more debris removal at 1 mm from working length than did needle irrigation.
One study is not in agreement with those positive outcomes discussed above. Jiang et al. ran a study and evaluated the EndoVac system’s ability to remove dentin debris from artificially made grooves in standardised root canals. The model was made of a single tooth root in which an apical groove comparable to an ovoid apical canal was created and packed with dentin debris. They compared several devices to activate the irrigation solution. Once the irrigation regimen was completed, they viewed the grooves through a stereomicroscope to evaluate the residual dentin debris. A score between 0 and 3 was given to each specimen: 0 = the groove is empty, 1 = less than half of the groove is filled with debris, 2 = more than half of the groove is filled with debris and 3 = the complete groove is filled with debris. The specimens irrigated with the EndoVac system had their groove completely filled with debris (score 3) 65 % of the time, while 35 % had less than half filled with debris [17]. It is important to note that Jiang et al. failed to follow the manufacturer’s instructions by failing to use the critical macrocannula, an error that could easily cause the microcannula to clog and become ineffective. When the microcannula is blocked by debris, the clinician will experience decreased or complete arrest of irrigant flow. To rectify the situation, the microcannula can be wiped with a 2 × 2 gauze or air and water can be blown into it to unclog it. This can also be done with the macrocannula should it also become clogged during its use (Fig. 9.10). Complete clogging of the microcannula happens very rarely, if the macrocannula is used according to the manufacturer’s instructions. The microcannula will continue to work even if several holes are blocked. However, its effectiveness will decrease. To avoid this complication, the macrocannula’s main purpose is to remove as much debris as possible before the smaller microcannula is introduced. This will reduce the incidence of it clogging as long as the macrocannula is used according to the manufacturer’s recommendation. A weaker capacity of the EndoVac system to remove apical debris could be attributed to the minimal turbulence intensity produced within the canal by the microcannula [28]. This evidence of low wall shear stress values causes a minimum physical interaction between the irrigant and the root canal walls [29]. This absence of interaction may explain the difficulty of the irrigation solution to reach the root canal’s lateral canals and anastomoses [5].


Fig. 9.10
If either cannula becomes clogged, try unclogging it by attaching the back end of either the fingerpiece or handpiece onto a syringe filled with water. Push the plunger; in most instances the hole(s) is immediately cleared (Courtesy Kerr Endodontics (SybronEndo). Orange, California)
Microbial Control
The effective removal of organic and inorganic tissues would logically allow better access and elimination of endodontic pathogens, responsible of apical periodontitis, localised in the root canal system.
Hockett et al. tested the ability of apical negative-pressure irrigation to remove a thick biofilm of E. faecalis in mesial roots of mandibular molars, finding that these specimens rendered negative cultures after 48-h incubation, while some of those irrigated using traditional positive-pressure irrigation were positive at 48 h [29]. One in vivo dog study found that apical negative-pressure irrigation with 2.5 % NaOCl resulted in similar bacterial reduction than the use of apical positive-pressure irrigation combined with seven days of intra-canal medication which was the triple antibiotic paste [30]. The triple antibiotic Trimix (metronidazole, ciprofloxacin and minocycline) has been utilised for pulpal regeneration/revascularisation in teeth with incompletely formed apices [31]. The antibiotic medication is applied in regeneration cases to safely kill bacteria. Since the triple antibiotic versus the use of EndoVac with NaOCl was statistically equivalent for mineralised tissue formation and the repair process [30], the study [30] suggests that EndoVac may overcome the need for intra-canal medication. Further research is required to evaluate this potential. Using apical negative pressure with NaOCl also decreases the risk of drug resistance, tooth discoloration and allergic reactions often seen with the administration of antibiotics [32, 33]. A recent randomised controlled clinical trial [34] compared the antimicrobial effectiveness of EndoVac system and the traditional positive-pressure syringe and needle for irrigation. From the 16 mandibular molar treated with the conventional method, negative culture was found in 67 % compared to 100 % among the apical negative-pressure irrigation group. A second clinical study [35] demonstrated a higher frequency of obtaining negative culture with EndoVac system compared to a syringe with regular needle. Unlike Cohenca et al. [34], Pawar et al. [35] did not reach significance between the two clinical groups. However, Pawar et al. added an overriding codicil in their discussion: “The original EndoVac protocol recommends using a concentration of 5.25 % NaOCl. Almost all studies investigating the efficacy of EndoVac have used NaOCl at concentrations ranging from 2.5 to 6 %. The use of 0.5 % NaOCl [a 1,000 % dilution from the manufacturer’s instructions] in this study could be considered responsible for the lack of significant differences in antimicrobial efficacy between EndoVac irrigation and standard irrigation” [35].
Smear Layer Removal
The smear layer is created when the dentinal walls of the root canal system interact with endodontic instruments [36]. The smear layer is comprised of inorganic and organic material such as dentin filings and pulp tissue remnants [37]. This deposit can be penetrated by bacteria and may offer protection to biofilms adhering to the root canal walls [38]. Furthermore, the smear layer interferes with the tight adaptation of currently used root canal sealers to dentinal walls and may therefore promote microleakage [39]. Torabinejad et al. [40] suggested that the removal of the smear layer decreases bacteria and improves adaptation of obturation materials to the canal walls. Another study showed that the smear layer produced during root canal preparation promotes adhesion and colonisation of P. nigrescens [41
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


