The anterolateral thigh flap was first introduced by Song and colleagues in 1984 as a septocutaneous perforator–based flap. Advantages of this flap include long pedicle length, large vessel diameter, large skin paddle, two-team approach, and minimal donor site morbidity. Though originally presented as a septocutaneous flap, it is now known that the anterolateral thigh anatomy is quite variable, with the majority of patients having musculocutaneous perforators. Nonetheless, the anterolateral thigh flap has become one of the most popular soft tissue flaps and serves as a “workhorse” in oral and maxillofacial reconstruction with greater than a 90% success rate.
Anatomy
The anterolateral thigh flap is well known for variability in its vascular anatomy. It most often receives its blood supply from perforating vessels of the descending branch of the lateral circumflex femoral artery ( Fig. 71-1 ). The artery is quite robust with a mean diameter of 1.5 to 2.5 mm. Its paired venae comitantes usually have a diameter ranging from 1.8 to 3 mm. The artery travels inferiorly in the intermuscular space between the vastus lateralis and the rectus femoris muscles ( Fig. 71-2 ). Several perforator vessels then supply the skin of the lateral aspect of the thigh, with the dominant perforator usually being centered along a midpoint between an imaginary line drawn from the anterior superior iliac spine to the lateral aspect of the patella. Although Song and associates originally described the flap as a septocutaneous perforator flap, based on anatomic dissection it is now known that the majority of the perforator vessels (80%) are musculocutaneous and travel through the vastus lateralis.
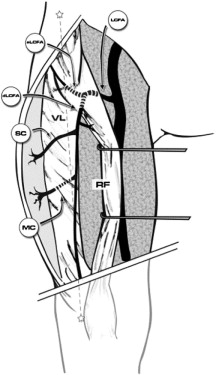
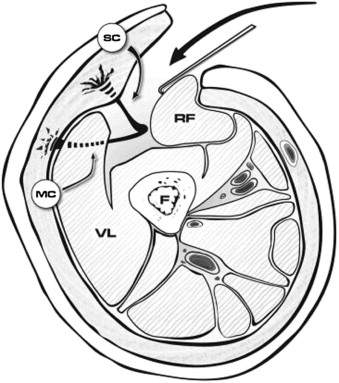
There are two common anatomic variations through which the musculocutaneous perforators traverse the vastus lateralis muscle. The more usual one involves a short course of approximately 3 to 5 cm through the muscle with origin from the descending branch of the lateral circumflex femoral artery. The second, less common variant of the musculocutaneous perforator involves an intramuscular course of approximately 6 to 7 cm, parallel to the vastus lateralis and branching from the transverse branch of the lateral circumflex artery or the lateral circumflex artery itself. In reviewing 37 consecutive anterolateral thigh flaps, Shieh and co-workers noted that the more common musculocutaneous course occurred in 57% of patients, with an intramuscular perforator length of 5 cm and a mobile pedicle length of 12 cm. A musculocutaneous course arising directly off the lateral circumflex artery occurred in 27% of patients, with an intramuscular perforator length of 7 cm and a mobile pedicle length of 11 cm. In a recent review of 89 consecutive anterolateral thigh flaps, Wong and associates described an oblique artery and associated paired venae comitantes, previously unnamed, traveling between the descending and transverse branches of the lateral circumflex femoral artery. This distinct artery was present in 35% of patients and had a mean diameter of 1.5 mm and pedicle length of 12 cm. The oblique branch was usually accompanied by the motor nerve to the vastus lateralis. Other vascular patterns do exist, including vessels arising independently from the profunda femoris or the trunk of the femoral artery, both of which occur less than 2% of the time. Absence of perforators has also been reported in 1% to 5% of patients.
The quadriceps musculature is composed of four muscles: the rectus femoris, the vastus lateralis, the vastus intermedius, and the vastus medialis. It is responsible for both knee extension via concentric contraction and knee deceleration via eccentric muscle contraction. The quadriceps femoris converges into the quadriceps tendon, which both attaches to and stabilizes the patella. The vastus lateralis muscle is the largest of the quadriceps muscles. It is innervated by motor nerves traveling within the intermuscular septum between the rectus femoris and vastus lateralis. These motor nerve branches, derived from the femoral nerve, are often intimately associated with the vascular pedicle of the anterolateral thigh flap. A review of 36 cadaveric thighs by Rozen and co-workers demonstrated four to seven motor nerve branches passing through the main vascular pedicle or between perforators in 31% of patients.
The anterolateral thigh flap derives its sensory innervation from the lateral femoral cutaneous nerve as it enters the thigh deep to the inguinal ligament just inferior to the anterior superior iliac spine. This nerve then divides into a larger anterior branch, which provides sensation to the anterolateral aspect of the thigh, and a smaller posterior nerve branch.
Diagnostic Studies
The greatest concentration of reliable perforators is located within a 3-cm radius at the midpoint between an imaginary line drawn from the anterior superior iliac spine to the ipsilateral lateral patella. Traditionally, no advanced studies have been required other than to preoperatively perform Doppler flowmetry and mark the skin perforators before making any incision. Various studies have reported that acoustic Doppler flowmetry has a 40% concordance rate with the intraoperative findings whereas color Doppler, though time consuming and ultrasonographer dependent, has a 96% to 100% positive predictive value. Frequently, one can make an incision along the pre-drawn line and carefully reflect the skin and fascia laterally until a perforator is identified. The flap can then be centered over this perforator. Computed tomographic angiography has also been used to assess perforator availability when selecting the anterolateral thigh as a possible flap donor site. Ribuffo and colleagues were able to determine perforator location and size and identify the septal/musculocutaneous pathway with computed tomographic angiography. The authors noted that all of the flaps survived and that preoperative imaging anatomy coincided with the operative findings. Disadvantages of the study included absence of a control group (handheld Doppler identification of perforators), cost of the imaging, and exposure of the patient to radiation. Preoperative angiography for the purpose of identifying significant vascular disease in the descending branch of the lateral circumflex femoral artery is unnecessary and of low yield because of the low incidence of atherosclerosis in this vessel.
Clinical examination is also of importance because previous surgeries on the thigh, including vascular bypass procedures, exclude the use of this flap. Large body habitus and excessive subcutaneous fat make both dissection and flap inset difficult.
Diagnostic Studies
The greatest concentration of reliable perforators is located within a 3-cm radius at the midpoint between an imaginary line drawn from the anterior superior iliac spine to the ipsilateral lateral patella. Traditionally, no advanced studies have been required other than to preoperatively perform Doppler flowmetry and mark the skin perforators before making any incision. Various studies have reported that acoustic Doppler flowmetry has a 40% concordance rate with the intraoperative findings whereas color Doppler, though time consuming and ultrasonographer dependent, has a 96% to 100% positive predictive value. Frequently, one can make an incision along the pre-drawn line and carefully reflect the skin and fascia laterally until a perforator is identified. The flap can then be centered over this perforator. Computed tomographic angiography has also been used to assess perforator availability when selecting the anterolateral thigh as a possible flap donor site. Ribuffo and colleagues were able to determine perforator location and size and identify the septal/musculocutaneous pathway with computed tomographic angiography. The authors noted that all of the flaps survived and that preoperative imaging anatomy coincided with the operative findings. Disadvantages of the study included absence of a control group (handheld Doppler identification of perforators), cost of the imaging, and exposure of the patient to radiation. Preoperative angiography for the purpose of identifying significant vascular disease in the descending branch of the lateral circumflex femoral artery is unnecessary and of low yield because of the low incidence of atherosclerosis in this vessel.
Clinical examination is also of importance because previous surgeries on the thigh, including vascular bypass procedures, exclude the use of this flap. Large body habitus and excessive subcutaneous fat make both dissection and flap inset difficult.
Treatment/Reconstructive Goals
The anterolateral thigh flap is ideally suited for reconstruction in the oral and maxillofacial region. It can be used for large soft tissue defects both extraorally and intraorally ( Figs. 71-3 and 71-4 ). It can be harvested as either a fasciocutaneous or a musculocutaneous flap, depending on the amount of soft tissue required. A true chimeric flap can also be elevated by combining the anterolateral thigh fasciocutaneous flap with the rectus femoris, vastus lateralis, and tensor fasciae latae. The flap is able to be thinned primarily in patients with excessive subcutaneous fat. The vascular pedicle is generally of good caliber and length, thus facilitating vessel anastomosis. A sensate flap based on the lateral femoral cutaneous nerve can be elevated if so desired. There is minimal donor site morbidity, and the anterolateral thigh flap can be raised in a two-team approach, which decreases surgery and anesthesia time.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


