Methods
Age range
Reliability
Authors (selection)
I. Tooth development
Development of several types of teeth
<ca. 14 years
<2 years
II. Dental morphology
Macroscopically (in situ)
All ages
Rough estimate only
Endris (1979)
Morphology of permanent teeth
Adulthood
±10–24 years ±10 yearscannot be reproduced
III. Biochemistry
1. Racemization of asparticacid in dentin
(a) Extracted teeth
All ages
±3–8 years
(b) In dentin biopsies
All ages
±6 years
Ritz et al. (1995)
19.1.1 ad 1: Stages of Dentition
For morphologic (anatomical) purposes, the following main stages of dentition are significant (Thoma 1953):
-
The time of formation of dental germ, development, and calcification
-
The time of eruption of milk teeth and permanent teeth
During the second dentition stage, between the age of 6 and 12 years, determination of age should not be based on only the eruption of permanent teeth. The progress of resorption of deciduous tooth roots up to the total loss and the progressive formation and calcification of the roots of the permanent teeth are helpful features and are displayed on the radiograph.
19.1.2 ad 2: Determination of Racemization of Aspartic Acid in Dentin
The aging phenomenon of intravital racemization of aspartic acid in human proteins was first described in the 1970s. Since then, the level of racemization of aspartic acid is used to determine the age of fossil materials (Pfeiffer et al. 1995). The racemization of aspartic acid in dentin is, compared with other methods, a very accurate measure for age estimation.
Like all amino acids, aspartic acid may occur in two forms: in a l-and in a d-form. In the biosynthesis of human proteins, exclusively l-amino acids are used. From the time of protein synthesis, a spontaneous, non-enzymatic conversion of l-aspartic acid residues into their d-form may occur. This in vivo racemization may lead to an age-dependent accumulation of d-aspartic acids in long-living, “aging” proteins, if they cannot be replaced after synthesis (they are permanent) (Ritz-Timme 1999). With advancing age, this accumulation is observed in bradytrophic tissues and gives objective information about the age of a person (Pfeiffer et al. 1995). In this respect, dentin tissue is the optimal tissue with its extremely bradytrophic metabolism and a relatively homogeneous and stable anatomic and biochemical structure. In forensic practice, the analysis of dentin is the standard method for biochemical age estimation (Ritz et al. 1990; Pfeiffer et al. 1995; Ritz-Timme 1999), because dentin analysis yields a wide range of basic data at a reasonable cost and has the advantage that it can also be used in examination of living persons (the examination of other kinds of tissues is reserved for special cases). In Germany, age estimation in living persons by this method was not applied until 1995 (Ritz et al. 1995). The main application is the adult age (Ritz-Timme 1999).
19.1.3 Methodology
-
Tooth preparation must be standardized (preferably by a dentist).
-
The prepared root dentin is washed in several steps.
-
The washed dentin is hackled (after freeze drying).
-
The resulting dentin powder is aliquoted.
-
The acid extraction is carried out with 0.6 N hydrochloric acid (the acid-soluble fraction is freeze dried).
-
This sample preparation results in a total dentin sample per tooth and an acid extract.
-
The total dentin sample and the acid extract are hydrolyzed and derivatized, and amino acids (as N-trifluoracetylisopropylester) are separated by gas chromatography on a chiral capillary column.
Dentin extraction is permitted only with active consent of the person, namely:
-
In extracted teeth
-
After tooth extraction with existing clinical indications
Otherwise, dentin biopsy will be performed. Dentin biopsy is legitimized in legal and social indications (Ritz and Kaatsch 1996). The testing laboratories must meet minimum requirements. A postmortem age estimation by the level of racemization of aspartic acid is possible with good results, because of a close relationship between the level of racemization and the age of root dentin, especially if a majority of coronal dentin is missing (Ritz et al. 1993a, b).
19.1.4 ad 3: The Radiological Investigation
According to § 24 RöV, a radiological investigation is permissible only if medically indicated and, according to § 81a STPO, permitted only by judicial decision. Following the principle of proportionality, a radiological investigation in suspected criminal offense (§ 81a STOP) may be performed by force, in contrast to asylum procedure, where empowerment is not given.
In this context, the dental panoramic radiograph (OPG) is based on the following technical parameters (Vendura et al. 1998):
-
OPG (orthopantomograph/radiograph): tube voltage: 63–81 kV
-
Maximum radiofrequency current: 9 A at 80 kV
-
Film size: 15 × 30 cm
-
Radiation exposure: dose equivalent 750–800 micro-Sievert. The radiation-sensitive organs (eye, thyroid gland) are outside of the useful radiation
Events, methods, and legal aspects of age estimation according to Ritz and Kaatsch (1996) are given in Table 19.2.
Table 19.2
Age estimation in living persons
|
Occasions
|
Methods
|
Legal aspects
|
|---|---|---|
|
Criminal responsibility of youngoffenders: >14 years old (§ 19 StGB)
|
Radiological assessment of the dentition
|
X-rays (§ 24 RöVO) only byjudicial decision (§ 81a StPO)
|
|
Young asylum seekers aged >16 yearsold (§16 of the Asylum ProcedureAct, § 68 of the Aliens Act)
|
Radiological assessment of thedevelopment of the 3rd molar
|
X-rays (§ 24 RöVO) only byjudicial decision (§ 81a StPO)
|
|
Applicability of the adult criminallaw for young offenders: >18 or 21years old? (§ § 1, 105 JGG)
|
Radiological assessment of thedevelopment of the 3rd molar (Thorson and Hägg 1991)
|
X-rays (§ 24 RöVO) only byjudicial decision (§ 81a StPO)
|
|
Determination of racemization of aspartic acid in dentin
|
Dentin recovery only witheffective consent
|
|
|
On extracted teeth
|
Tooth extraction only with existingclinical indication, otherwise dentin biopsy
|
|
|
In dentin biopsies
|
Legitimized dentin biopsies with“legal and social” indications
|
|
|
Clarification of pension claim
|
Determination of the racemizationlevel of aspartic acid in dentin
|
Dentin recovery only with effective consent
|
|
On extracted teeth
|
Tooth extraction only with existingclinical indication, otherwise dentin biopsy
|
|
|
In dentin biopsies
|
Legitimized dentin biopsies with“legal and social” indications
|
In cases of suspicion of an obvious abuse, whether the suspect is older or younger than 16 years (if no other data is available) is important, and thus an investigation for age estimation is legally admitted. The legal occasions for age estimation of living individuals are usually for criminal, administrative, civil, and social issues (Ritz and Kaatsch 1996):
Criminal law
-
Criminal responsibility – age >14 years – § 19 StGB
-
Applicability of adult criminal law – age > 18 or 21 years – §§ 1, 105 AJC)
Administrative Law
-
Asylum – § 16a para 1 GG, § 12 para 1 and 3 AsylVfG
-
Ability to submit applications and declarations, Aliens Act – § 68 age estimation without or with invalid identity documents
Civil Law
-
Changes of age in the family book – § 47 PStG
Social Law
-
Clarification of pension claims of foreign citizens whose date of birth is not or allegedly not known – §§ 35, 33a SGB VI
19.2 First Dentition (Deciduous Dentes)
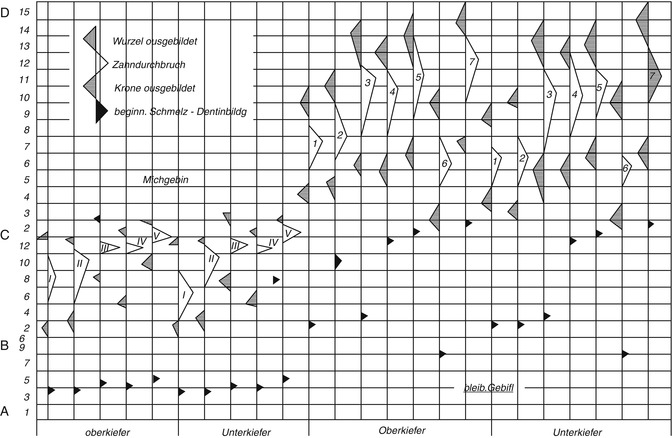
Tooth development can be divided into four stages (Gustafson and Koch 1974):
1.
Root development
2.
Tooth eruption
3.
Crown formation
Every stage of the eruption is declared (the tip of the triangle corresponds to the average), and the largest variation is also declared (the legs of the triangle point to the respective time limit of the eruption). Gender differences are not taken into account.
Many forensic odontologists use the scheme (Figs. 19.2 and 19.3) developed by Schour and Massler (1958) and varied by Ferembach et al. (1979). The fact that the data originated from children who have died from diseases is not considered here. For this reason, the table of the chronology of dental development and tooth growth by Schour and Massler (1940) is of limited suitability (Rötzscher and Reimann 1975). Gender differences are also not included. The schemes are based on the work of Logan and Kronfield (1933) and are often quoted. The American Dental Association (ADA) periodically updates and publishes this table.
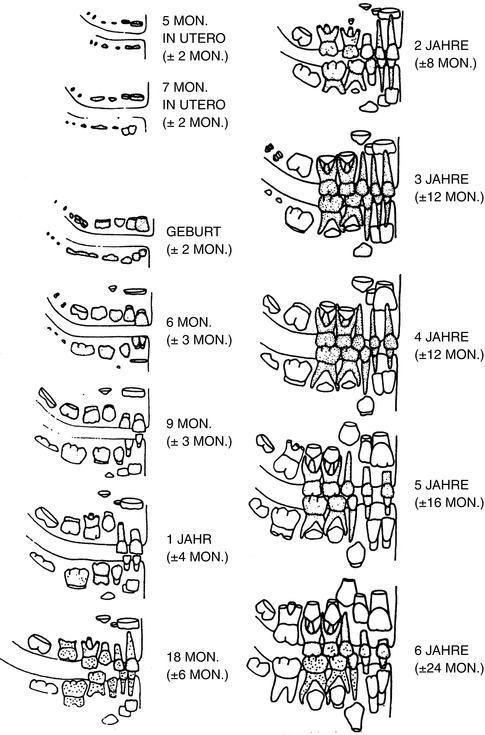
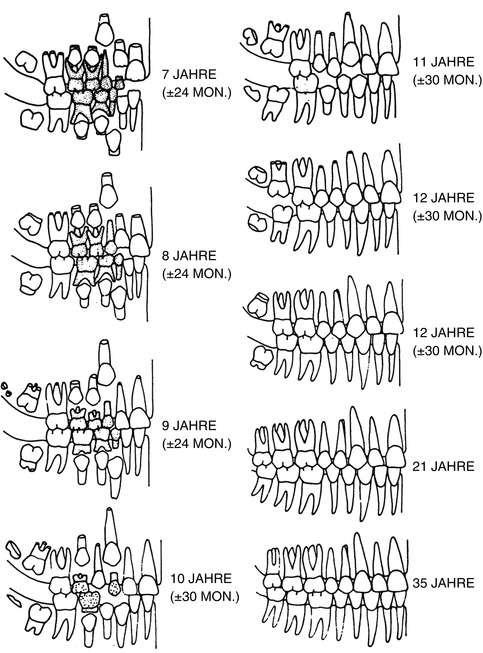

Fig. 19.2
Milk teeth (deciduous dentes)

Fig. 19.3
Permanent teeth
Ciaparelli (1985) compared the data with subject groups (schoolchildren) and found that the information corresponded with boys at the age of 4–16 years; in girls this development takes place 3–6 months earlier. The variables were similar at the age of 4–6 years; in boys at the age of 12 years, they doubled, and they tripled at the age of 16 years. Rösing (1994) used this scheme as does Ferembach et al. (1979) (see Figs. 19.2 and 19.3).
19.3 Second Dentition (Permanent Teeth)
In contrast to the deciduous tooth eruption, the eruption of the permanent dentition extends over a time period that is much longer with many more irregular intervals. In addition, the eruptions have considerable fluctuations, especially the last erupting teeth (Künzel 1976).
Figure 19.4 shows the variability of age groups where certain stages of development are reached (Moorrees et al. 1963). Average ages with a single (+1) and double (+2) standard deviation (s) are shown, in which the complete crown formation (Kk), certain fractions of the root length (1/4, 1/3, 1/2, 2/3, 3/4), total root length (Wx), and the apex half or full circuit (A1/2 k) of the permanent upper and lower central (I1) and lateral (I2) incisors are achieved (black symbols and lines = boys, red = girls) (Moorrees et al. 1963).
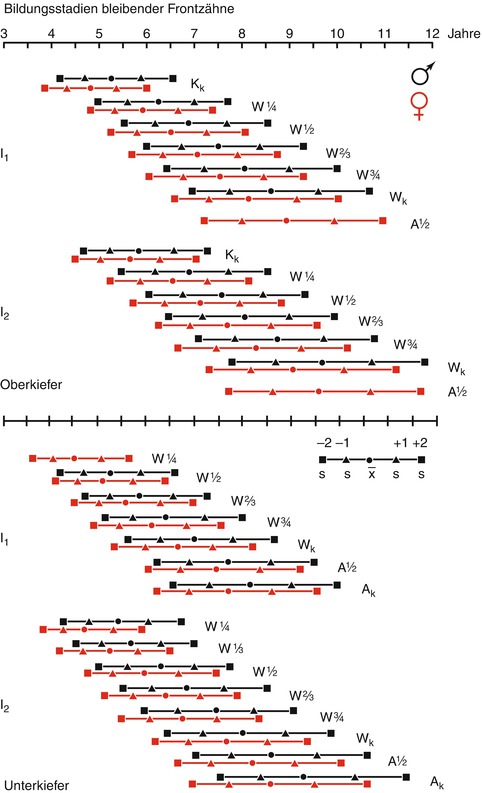

Fig. 19.4
Formation stages of permanent teeth
Most effective is a classification of the tooth development in as many stages as possible. Moorrees et al. (1963) divided the stages into 14 steps, but the tables are difficult to read.
Dental development is very regular and only slightly dependent on diet, hormones, and disease (Crossner and Mansfeld 1983). Hormones such as growth hormone can affect tooth development, but less than bone development (Garn et al. 1965). Children in warmer regions develop earlier (~6–14 months), according to a study of Arabian children by Fahmy (1974). In an Indian investigation, Kumar and Sridhar used (1990) the Probitt analysis for the eruption and, thus, could calculate the probability for each deviation: up to the age of 9 years, the discrepancy is approximately 1 year, and a little longer up to the age of 13 years. After 13 years, attention must be paid to the wisdom teeth, which have a greater range of variation, of approximately 2–3 years (Haavikko 1974). Note that the dental age may differ from the “chronological” age (Hurme 1957; Schranz 1958, 1959).
The first molar tooth is the first tooth of the permanent teeth that erupts at the end of the milk teeth; it is also called the 6-years molar, because of its eruption at the age of 6 years. The first phase of the dentition is complete with the eruption of the first molars and the permanent incisors (early mixed dentition) between the age of 6 and 9 years. The eruption of the premolars, the canines, and of the second molars takes place in the second phase, between the age of 9 and 13 years.
Adler (1957a, b, 1959a, b, 1963) collected the eruption events in the permanent teeth in a Hungarian subject group and according to international literature. Estimating dental age in children and young people of foreign origin exclusively by their teeth is difficult, as the tables by Adler (1957a) show. Both the date of the eruption and the frequency of the occurrence of changes in the number of teeth are variable (Hägg and Matsson 1985). Thus, premature extractions of primary molars and molars (Rönnermann 1977; Hägg and Matsson 1985) may result in premature eruption of posterior teeth. The standard deviation in age estimation for the number of teeth according to Helm (1990) is 1 year, in 5 % of all cases an age difference of up to 2 years is expected.
According to several authors, conclusions regarding age are not reliable because the number of teeth is a variable criterion (Liliequist and Lundberg 1971; Preece 1983; Towlson and Peck 1990). A literature research by Marré and Hetzer (1999) shows the ages (average in years) of tooth eruption.
An age estimation (based on the third molars and on the remarks by Demirjian) of 823 white US citizens was conducted by Mincer et al. (1993). The authors declare the regression equations for age estimation by reference to the third molars, but they also point to the marked variability in the development of third molars. Demirjian et al. (1973, 1976) describe the eight stages of mineralization. Each stage is described by a scale value. The values are added and assigned to a certain age (Demirjian 1994). The authors quote differences of up to 3 years from the actual (from 3rd to 97th percentile).
Haavikko (1974) modified this method by assigning 12 mineralization stages to four teeth, always six for the root and the crown. The values are added and divided by the number of the assessed teeth and so the age is determined (accuracy of ±2.3 years between the 10th and 90th percentile).
Liliequist and Lundberg (1971) have differentiated seven development stages of a tooth. Each stage also receives a scale value here. The values are added and assigned to an age with the help of an age table.
The most widespread method in practice probably is the method by Demirjian. Davis and Hägg (1994) have applied the method by Demirjian et al. (1973) in 975 Chinese children of preschool age, taking into account the standards of 1982 and 1986. The most distinctive deviations between actual and estimated age occurred in the older age groups, and were more frequent in boys than in girls.
Staaf et al. (1991) compared the accuracy of these three methods in 514 Swedish children from 5.5 to 14.5 years. They noted that in the application of the study by Demirjian et al. (based on Canadian values) for a Scandinavian subject group, the age was overestimated by an average of 6–10 months. The 95 % confidence interval was, depending on the method, up to ±2.1 years.
A new approach for age estimation was developed by Mörnstad et al. (1994). Based on orthopantomograms of 541 Swedish children from 5.5 to 14.5 years, the following parameters were digitized, metrically recorded, and mathematically and statistically elaborated: canopy height (coronal height) width, the distal apical foramen, and the mesial and distal root length of molars, premolars, and single-rooted teeth. A multiple correlation model was developed that provides values that are more precise than other methods, with an accuracy of ± 1.5 to 2.0 years in the 95 % confidence interval.
Tooth development is initiated in the fifth embryonic week (after ovulation). However, even the wisdom tooth, whose germ is formed several years after birth and whose crown is completed before the middle of the second decade of life, is formed by the same biological rules as the prenatal development of deciduous teeth (Schroeder 1987). The third molar, the so-called wisdom tooth, is the most flexible tooth in the permanent dentition, but there are situations in which the third molar is the only available age criterion.
Otuyemi et al. (1997) evaluated the eruption times of third molars in 1,071 13–21-year-old children and young people from rural areas of Nigeria. Of the 4-year-old children, 1.1 % already had all four wisdom teeth. The eruption took place at the average age of 14 years in boys and 13 years in girls. The eruption of the third molar is earlier in contrast to European and American values. This fact is, according to the authors, attributable to nutrition that is more fibrous. Ajmani et al. (1986) detected similar values for the time of eruption in a study of 1,238 15–21-year-old children and young people from northern Nigeria, but the average age was found to be 18 years. Hassanali (1985) determined in (a) 1,343 African and (b) 1,092 Asian students in Nairobi, Kenya, that the eruption time of the third molar was 17.6–18.9 years for (a) and 19.9–21.0 years for (b). This difference is significant.
Thorson and Hägg (1991) applied the method by Demirjian (1986) to 372 adolescents with ages from 14.5 to 24.5 years. In girls, the age of the 14- to 17-year-old girls was underestimated by 6 months, and in 20- to 25-year-old women, by up to 3.5 years. The standard deviation in all age groups was approximately 24 months. In male subjects, ages younger than 20 years old were underestimated by 10–17 months. In the 20-year-old men, the root development was already completed in 33 out of 34 subjects, found that 10 % of the root growth of third molars is already completed at the age of 17 years.
From the age of 16 years, the number of subjects with completed root development increases. Because of agenesis of the third molar, in 20 % of the cases, conclusions about the development state are not possible. Thorson and Hägg (1991) note, that based on the development of the third molars, a definitive statement regarding the age of 18 years is not possible. They refuse an age estimation for legal purposes that is based on the development of the third molars.
The available data in the international literature shows that the average time of tooth eruption varies in different ethnic groups. A blanket application of the values for whites to other ethnic groups is not possible. The determination based solely on the third molars should only be performed if no other criteria are available.
Interdisciplinary cooperation for age assessment in a consortium of experts is required and desirable to provide a quality assurance system. Age estimation should not be made solely on the basis of tooth development, but rather in collaboration with anthropologists, orthodontists, pediatricians, forensic scientists, and radiologists, especially if criminal liability (Schmeling et al. 1999a, b) (age > 14 years – § 19 StGB) must be assessed.
The skeletal development of the hand and wrist (representative for the entire skeleton) in the X-ray image can be used as pars pro toto and provides information about the skeletal age (Greulich and Pyle 1988). The limit of this method is reached at the age of 18 years. Together with the panoramic radiograph, it can limit the range of variations of the possible age of the child/youth from different ethnic groups.
Cavalli-Sforza et al. (1994) divide the genealogical relationship of the world population into four main groups: Africans, Australians, Europeans (whites), and Mongolians. Greulich and Pyle (1988) apply the so-called Atlas method. It is the most common method for determining skeletal maturity (Cole et al. 1988; Milner et al. 1986).
However, ethnicity is not the only influence on the development of a child. Rather, it is believed that genetic factors are also responsible for the potential of skeletal maturity. A low socio-economic standard of living and poor environmental conditions lead to retardation (development delay) of skeletal maturity (Schmeling et al. 1999a, b).
For adults, the time-independent level of racemization of aspartic acid can be especially assessed, whereby the reconditioning of a tooth (otherwise a dentin biopsy) is necessary (Rösing and Kvaal 1998).
Investigations by Schmeling et al. (1999a, b) have demonstrated that skeletal maturation takes place in the same (identical) levels in all major ethnic groups. The risk of an age estimation below the actual age should serve to protect that person.
If a tooth is completely developed, all age-related changes at the tooth structure must be studied (Solheim 1988b). Often, they are described as degenerative or regressive, and attrition, color, and periodontal shrinkage are assessed by the naked eye. Although there is a correlation between age and size of the pulp chamber, it is not significant enough for age estimation (Prapanpoch et al. 1992). The tooth structure is subject to physiological and pathological influences and occurs as a consequence of tooth loss.
The main causes for hard tissue loss are:
-
Chemical–bacterial processes of decay
-
Extradental and intradental absorption rather than cellular processes
-
Factors leading to abrasio dentis (Schneider and Hampel 1966); dental abrasion is an important and indispensable finding
Interstitial friction surfaces (adjacent teeth in the middle decades of life), changes in the interior of the tooth (increasing narrowing of the lumen in crown-pulp-cavum and the root canals) and the alveolar process (atrophy with exposure of cervical and coronary artery root parts) provide valuable information.
From the statistical view, forensic age diagnosis is a bivariate problem with the two variables being signs of maturity and chronological age. Of great importance is the scattering of the bivariate distribution area (Riepert and Rittner 1989).
The studies of Gustafson (1947, 1950, 1955, 1956) provided the first statistical methodology. The “Gustafson method” was the basis of age estimation by teeth and hand. He assessed six factors of changes of dental hard tissue (Figs. 19.5, 19.6 and 19.7):
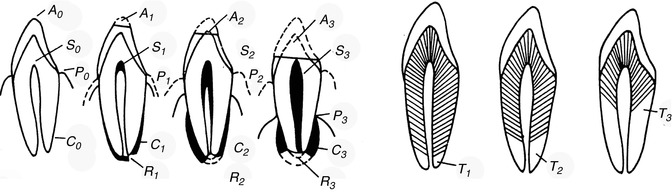
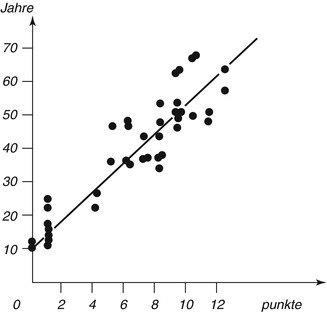
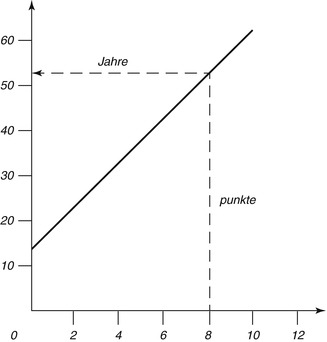

Fig. 19.5
Tooth grinding – six factors (changes in dental hard substance)

Fig. 19.6
The regression line between observed values is marked

Fig. 19.7
The regression line to determine the age of an unknown
1.
A = attrition (abrasio dentis)
2.
S = residue of secondary dentin in the pulp chamber
3.
P = periodontal shrinkage
4.
Z = cement residue
5.
R = cement and dentin resorption
6.
T = root transparency
The changes are microscopically examined on thin sections of slides embedded in canada balsam. The strength of each change is divided into four stages (0–3). Points are dependent on the degree of change, and adding all points of a tooth results in the sum of the changes (An + Pn + Zn + Sn + Rn + Tn = total). The relation between scores and age is expressed by a line (chart). The calculated formula is: age = 4.56x + 11.43. The correlation with age is 0.91 (Maples and Rice 1979).
The accuracy of the determination is increased if two or more teeth are available. Then the average of the teeth is used as a total. The Gustafson method has the advantage that it evaluates several features, in comparison with a single feature, which is more error prone. Although several improvements have been published, e.g., in the USA, many forensic odontologists still use the original method. Thus, the Gustafson method was applied for many years as the method of choice by many forensic odontologists.
Nevertheless, this method is criticized (Schmeling et al. 1999a, b):
-
The evaluation of the points is the result of a subjective selection of the changes; it is based on too many factors that are pathologically influenced and not only related to age.
The examined material of 40 teeth of different individuals (Gustafson 1947, 1950, 1955) is a relatively small selection and it may be possible that some teeth are from the same individual, as in other investigations (Burns and Maples 1976; Johanson 1971; Lode and Reimann 1985).
Because the variability of age-related changes in the teeth from the same individual is apparently less than in teeth of different individuals, this can contribute to a more favorable standard deviation.
Gustafson (1947, 1950, 1955) developed a single formula that should be valid for all groups of teeth. However, it must be considered that age-related changes can lead to different results (Maples 1978; Schulze 1982) as much as tooth position (Schulze 1982), or a formula separately calculated for each tooth type (Bang and Ramm 1970; Schulze 1982). The regression formula by Gustafson was incorrect. Later it was amended to: age = 13.45x + 4.26 and the correlation between estimated and actual age r = 0.912 (Maples and Rice 1979). Nevertheless, it is not appropriate to skip Gustafson’s method and not to mention its results.
Biedow (1963) modified the Gustafson method by assessment of one-sided cut teeth under the stereomicroscope with the same scoring as Gustafson. It is recommended that X-rays be obtained before grinding in order to avoid errors in the assessment of regressive changes, because not every root canal is straight.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses