CONSTANTIN VÂRLAN
BOGDAN GALBINASU
Chapter VII
ADHESIVE TECHNIQUES IN ESTHETIC DENTISTRY
7.1.1 Introduction
Nowadays, there is almost unanimous agreement that bonding agents and procedures are the greatest breakthrough in the entire history of restorative dentistry. They have played a crucial part in the contemporary implementation and advancement of the increasingly popular esthetic aspects related to this field.
Adhesion to dental tissues, as well as to synthetic materials such as porcelain and polymer structures (resins), and even metal alloys, has revolutionized the principles and methods of current practice.
A comprehensive understanding of the phenomena taking place at the bonding interface of adhesive restoration requires a good knowledge of surface physical processes, such as surface tension and energy, wettability, and capillarity, as well as of those related to rheology, such as viscosity, flow, and thixotropy.1,2 The practical applications of these are to be found in various characteristics of the materials, which have a varying impact on their clinical behavior. There are various mechanisms which, if understood, may allow us to make the best use of specific materials in different clinical situations for which they are required and for which they have been created. These include adhesion of bonding agents, dental structures, micro- or nanoinfiltration of gaps or small enamel fractures around the dental restoration, etc.2
Adhesive bonding is the attraction between two surfaces in very close contact due to intermolecular forces that act at a very small distance. The materials able to bond two surfaces are called adhesives, and the material on which the adhesive is applied is called the adherent or substrate.
There are several types of adhesion, depending on the nature of the forces that keep two surfaces together:2
• Mechanical adhesion: present between rough surfaces due to mechanical friction. When the height of a roughness profile is at micron level (10-6 m), the physical process is called micromechanical adhesion and the respective roughness is called micromechanical retention.
• Electrostatic adhesion: occurs due to the attraction between opposite electrical charges.
• Dispersive adhesion: occurs due to bonds formed when the adhesive wets the substrate, resulting in a physicochemical adsorption. The intermolecular entanglement is the main force determining the adhesion: van der Waals forces (Keesom force, Debye force, London dispersion force), hydrogen bonding.
• Chemical adhesion: when the superficial atoms of two surfaces form ionic, covalent, or coordinate bonds.2,3
Mechanical, dispersive, and chemical adhesion are the foundation of bonding procedures in dentistry, leading to achievements impossible to reach with traditional techniques based only on geometric mechanical retention. They allow treatment in much more diverse clinical situations, with a better prognosis in time and, most obviously, with superior esthetic results.2,4
Mechanical interlocking also enables the adhesive to penetrate into the microscopic or submicroscopic irregularities of the adhered surface of a substrate. This type of bonding is known as micromechanical adhesion.3,5
The essential condition necessary in order to achieve a strong and durable interface bond is to have a permanent and as close as possible contact between the surfaces of the adhering bodies. In such cases, mechanical adhesion is accompanied by dispersive adhesion due to intermolecular attractions. The latter forces are efficient only if the distance is very small, ie, 1 to 2Å (1Å = 10-10 m). Consequently, when the conditions are right for the molecules of the adhesive to come into intimate contact with the molecules of the adherent at such a small distance, intermolecular forces of attraction emerge at once and determine the adsorption of the adhesive to the substrate, at the same time achieving its adhesion.2–4
7.1.2 Brief history
The first attempt at tooth bonding was made by Hagger who, in 1949, developed an adhesive system based on glycerophosphoric acid dimethacrylate, which he used to seal the margin and “stick on” the cavity walls. It is associated with a self-curing acrylic resin for crown restorations.
But the modern era of adhesive dentistry was initiated in 1955 by Buonocore, who was inspired by the methods used in the naval industry to improve the adhesion of paint and glaze to metal surfaces. He suggested that tooth enamel should be treated with phosphoric acid (at an initial concentration of 85%) in order to improve its bonding with the restorative resins.6,7
Acid etching became really useful a few years later, in 1962, when Bowen introduced the first effective resin-based dental composite called Bis-GMA. He was inspired by Knock and Glenn who, in 1951, suggested incorporating filling ceramic particles into the resins in current use at that time.8
In 1966, using the “Bowen-formula resin” (Bis-GMA) as a starting point, Newman and Sharpe developed a new composition with a much lower viscosity. They achieved this by eliminating the entire ceramic filling from the initial structure, thus creating the first dental adhesive.
Owing to its high efficiency and the low risk of technical errors, the procedure of acid etching of tooth enamel to increase the bond strength with the composite resin has continued to be used, although it has been subject to some changes in principles or techniques, such as decreasing the phosphoric acid concentration from 85% to 30–40% and the etching time from 40–60 to 15–20 seconds, as well as the use of etching products in the form of gel, etc.
Attempts at developing adhesive systems for bonding to dentin have resulted in the availability of a large range of materials and procedures, and research is being carried out even today. Topographic variety, chemical composition richer in organic substances and water, and the presence of dentinal fluid or of the so-called “smear” layer (discovered and named in the literature by Boyde in 1963) are some of the obstacles and challenges people have faced when trying to achieve an efficient bonding to the dentinal substrate.9,10
In 1970, Eick et al were the first to identify the chemical constitutive elements of the microscopic “smear” layer that covers the dentin after cavity instrumentation. In 1984, Brännström divided the layer into two parts, depending on their location: “smear on” – the part located on the dentin walls – and “smear in” – the extension of the former into the dentinal tubules. Nowadays, the most popular terms used in the literature are “smear layer” for the detritus film on the dentinal surface, and “smear plugs” for the material packed into the dentinal tubules (such as “corks” or “plugs”).6,11,12
The almost unanimously acknowledged attribute of the smear layer to protect the pulp-dentin system by decreasing dentin permeability led the specialists to initially ignore Fusayama’s suggestion (1980) to acid etch not only the enamel but also the exposed dentinal surfaces. This very controversial procedure was finally accepted, which resulted in “total etch” (or total acid etch).13
In 1952, Kramer and McLean made the first remarks concerning the presence of a hybrid layer when they noticed that the product developed by Hagger in 1949 had a tendency to penetrate the dentinal surface and form an intermediate area between the dentin and the restorative material. In 1982, Nakabayashi studied the characteristics of the hybrid layer, thus laying the foundation of the dentin hybridization theory. The formation of the hybrid layer eliminates the risk of marginal microinfiltration (regarded as the main source of pulpal complications) and leads to an adhesive “attachment” between the substrate and the restorative material.14
The next crucial step regarding adhesion to the dentinal substrate was the introduction of bonding agents with self-etching primer, meant to prevent nanoinfiltration phenomena at the base of the hybrid layer, as well as postoperative pain or tooth sensitivity. Most manufacturers focused on this idea to simplify clinical procedures, namely to shorten the time required for resin application, and to prevent practice errors. Irrespective of the aforementioned advantages, the impact of bonding agents with self-etching primer on the quality of bonding to enamel is still under discussion, principally regarding the bond strength to the substrate.15
An analysis of the crucial moments in the history of bonding in restorative dentistry reveals, on the one hand, the constant attempts by scientists and biomaterials manufacturers to improve products and techniques in response to clinical practice demands, and, on the other, the fact that the research is far from over. The aim is to develop and introduce on a large scale the so-called “all-in-one” or “universal” adhesive, a single adhesive able to offer simple and efficient adhesion solutions for all kinds of substrates.
7.2 ADHESION TO HARD DENTAL TISSUES
7.2.1 Strategies in approaching adhesion to hard dental tissues
The fundamental principle of adhesion to dental tissues is based on an exchange process by which inorganic dental material with a crystalline mineral structure is replaced by synthetic resin. The process involves two stages.16
The first stage (called “acid etching”) consists of the demineralization and removal of the calcium phosphate that makes up the hydroxyapatite crystals of the superficial layer, which results in microporosities on the surface of both enamel and dentin (Figs 7-1a to 7-1d).17,18
The second stage (defined as “hybridization”) consists of infiltration and in situ polymerization of monomeric resins within the created etch pits. In this way, bonding to the substrate is achieved through micromechanical interlocking that is chiefly based on mechanisms of diffusion. The latter aspect is regarded as a crucial sine qua non condition to achieving good bonding within clinical circumstances. The potential benefit of the additional chemical interaction between bifunctional monomers and tooth substrate constitutive elements has always been a main theoretical and practical concern, and is still a topic of contemporary research.
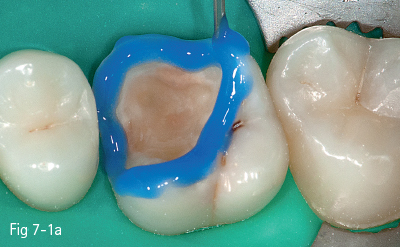
Fig 7-1 a Tooth 26: Acid etching of enamel.
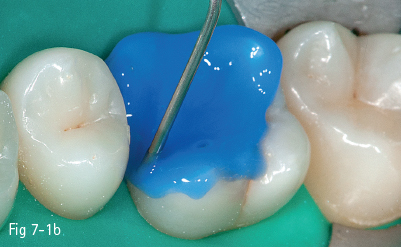
Fig 7-1 b Acid etching of dentin.
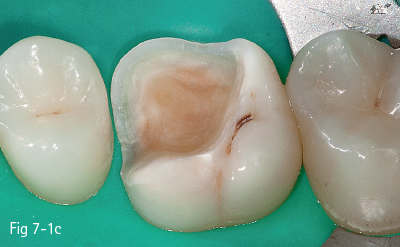
Fig 7-1 c Post-etching clinical view.
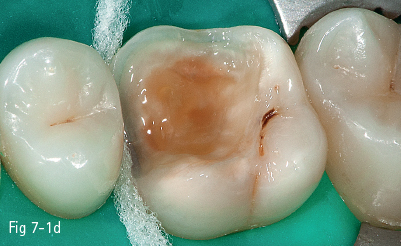
Fig 7-1 d Post-bonding view. (Images courtesy of Dr Ionut Brânzan.)
In modern enamel/dentin bonding systems, the adhesive interface with the substrate is achieved by following several “steps”, which also represent a classification criterion for adhesives. In addition to the clinical “steps” (of which there could be three, two, or only one), another essential criterion for defining and classifying bonding agents is the adhesion “strategy” underlying the therapeutic approach. In this regard, there are three classes of adhesives:20,22,24,64
• “etch-and-rinse”
• “self-etch”
• “glass-ionomer”.
There are substantial differences between them concerning the degree of substance exchange and the way in which the exchange takes place at the interface between adhesive and tooth substrate (enamel/dentin, or possibly cement). In general, the exchange intensity induced by etch-and-rinse adhesives exceeds that of self-etch adhesives;19 nevertheless, among the latter there are currently some adhesives that intensely interact with the substrate, even when only applied in “one-step”.22,23
7.2.1.1 “Etch-and-rinse” bonding strategy
This technique involves the application of the acid etchant as an initial, separate step, followed by substrate rinsing and water removal. The next steps consist of applying the primer, which is the diffusion-promoting agent indispensable to achieving adhesion, and finally the resin, which is the bonding agent proper. Thus, the etch-and-rinse strategy involves three separate steps, or two in the case of the simplified version when the primer and the bonding agent are mixed. The invariable and characteristic step is the compulsory and separate acid etching followed by rinsing.
The approach, which basically only requires two steps, is still the most effective for achieving solid and stable bonding to enamel. Selective dissolution of hydroxyapatite crystals through etching (the most common is 30% to 40% phosphoric-acid gel) produces microporosities on the substrate surface, where the resin absorbed by capillary attraction is polymerized in situ and envelopes the remaining exposed crystals. The resin, infiltrated within the prismatic structures of the enamel, interlocks with the etch pits in two ways, forming macro- and microresin tags. The macro tags fill the space surrounding the enamel prisms, while the more numerous micro tags, which result from resin infiltration and polymerization within the small etch pits at the core of the etched enamel prisms, play a major contributory role in the micromechanical bonding between adhesive and enamel.24,25
With regard to dentin, the phosphoric-acid treatment exposes the collagen network that is nearly totally “depleted” of hydroxyapatite crystals, thus displaying numerous microporosities within which nearly all calcium phosphates have been dissolved and removed. Consequently, the primary bonding mechanism of etch-and-rinse adhesives to dentin is first and foremost diffusion-based, and depends on the (as complete as possible) infiltration of resin within the exposed collagen fibril scaffold. Chemical bonding is rather unlikely and difficult, because the functional groups of monomers have less affinity to the hydroxyapatite-depleted collagen. This weak chemical interaction might be the main reason for the high long-term failure rate of the bonding between such adhesives and dentin.18,24,25
The main drawback of this technique is the significant delay between the moment when the microporosities are formed by acid etching and the moment when the monomeric resin infiltrates these gaps. In this time period, the conditions for filling the etch pits by diffusion must be created and maintained.
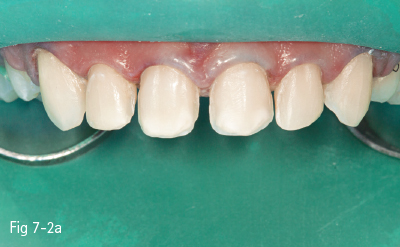
Fig 7-2 a Pre-acid etching clinical status.
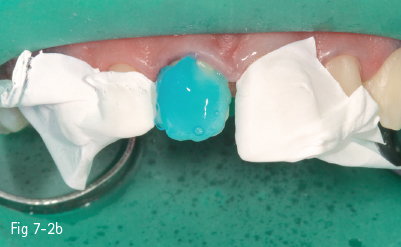
Fig 7-2 b Etchant applied on tooth.
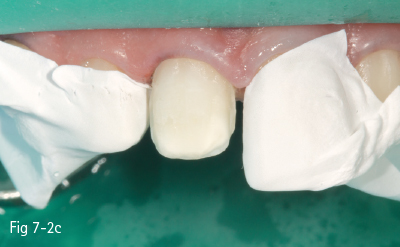
Fig 7-2 c Demineralized surface.
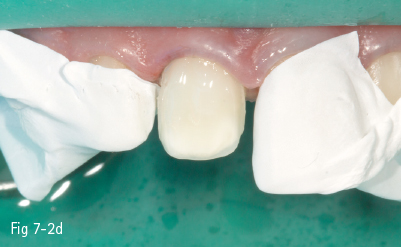
Fig 7-2 d Post-bonding view of the surface. (Images courtesy of Dr Florin Lazarescu.)
Consequently, the most critical step in the etch-and-rinse approach is the priming step. There are two techniques: dry-bonding and moist/wet bonding, depending on the solvent-based primer. Dry-bonding involves air-drying (not dehydration) of the acid-etched and washed dentin or enamel, followed by priming with a water/ethanol-based hydrophilic primer. Wet-bonding involves removal (preferably by absorption) of excess water after rinsing the acid-etched tooth, followed by the mandatory use of a hydrophobic and highly volatile acetone-based primer (Figs 7-2a to 7-2d).
Both techniques involve “delicate” technical issues and there is a risk of errors during application and manipulation of the substances, which could lead to either deterioration of the hybrid layer, resulting in loss of marginal seal integrity (opening the way for nanoleakage), or postoperative pain, because of the dentinal fluid movement within dentinal tubules.23,26
7.2.1.2 “Self-etch” bonding strategy
Clinically, the self-etch bonding strategy is the most promising regarding user-friendliness and the reduction of technique-sensitivity. In such an adhesive system, low-pH etchers (functional monomers with one or more carboxylic or phosphate acid groups) are mixed with a self-etch primer. As no separate acid etching step followed by rinsing and drying is required, the clinical application time is reduced, and the risk of errors during application and manipulation is lowered.27–29
The self-etch approach involves two steps, or a single step in the case of the simplified version when the primer and the bonding agent are mixed. What is peculiar to this technique is the fact that the mandatory separate etching stage followed by rinsing is no longer present.30,31
As the infiltration of resins occurs simultaneously with the etching process in this bonding strategy, the problems related to the delay between the emergence of etch pits following acid etching and monomeric resin infiltration within the gaps are largely diminished (if not completely removed).
On the other hand, there is still the question of the long-term effects of incorporating dissolved hydroxyapatite crystals and residual smear-layer remnants within the bond. At the same time, it is important to know how much of the self-etching primer/adhesive solvent is retained within the interfacial structure. A solvent surplus will directly weaken the bond integrity (diminishing the bond strength at the substrate) and provide channels for nanoleakage, or it may inhibit the polymerization of the infiltrated monomers. Another significant aspect is related to the fact that the resultant interfacial structure is much more hydrophilic and, consequently, more prone to hydrolytic degradation.
Self-etching acid monomers have various degrees of pH level. Depending on the etching aggressiveness, self-etch adhesives can be subdivided into two categories: strong and mild.32
Strong self-etch adhesives usually have a pH of 1 or below. This high acidity results in a rather deep demineralization effect.
At the enamel layer, the resulting acid-etch pattern rather resembles the phosphoric-acid treatment following an etch-and-rinse approach, but is less deep and, consequently, less efficient with respect to the bond strength at the enamel layer by means of mechanical microinterlocking.
At the dentin layer, collagen is exposed and nearly all hydroxyapatite crystals are dissolved. Consequently, the underlying bonding mechanism is chiefly diffusion-based, which is similar to the etch-and-rinse approach. Low-pH self-etch adhesives have often been documented with rather low bond-strength values at dentin. Besides the high initial acidity that results in a weakened bonding performance, another problem is the effect of the residual solvent (water) of the self-etch primer that remains within the adhesive interface. The latter cannot be completely removed, which calls into question the long-term stability of this strong self-etch approach.30,31,33
Mild self-etch systems generally have a pH of around 2. On dentin, the demineralization is rather low, only to a depth of 1 µm. This superficial demineralization occurs only partially, keeping residual hydroxyapatite crystals still attached to the collagen network. Even under such circumstances, they produce enough etch pits to obtain micromechanical interlocking through hybridization. Although the hybrid layer is thinner than that produced by the strong self-etch, not to mention the etch-and-rinse approach, it does not have a decisive impact on the actual bonding effectiveness. The preservation of some amount of hydroxyapatite within the submicron hybrid layer may serve as a receptor for additional chemical bonding. Carboxylic acid-based monomers, such as 4-META, and phosphate-based monomers, such as phenyl-P and 10-MDP, have a chemical bonding potential to calcium of residual hydroxyapatite.34,35
There is a theory according to which a weak self-etching effect is desirable and useful because it solves several problems simultaneously: dealing with the smear layer that results from cavity preparation, achieving micromechanical interlocking within etch pits at the enamel layer, and producing a thin hybrid layer by infiltration within dentin etch pits.
Micromechanical retention is mandatory in order to resist acute debonding forces at the adhesive interface. The exposed hydroxyapatite enamel surface and the hydroxyapatite crystals that remain around collagen in dentin (in the case of mild self-etching) offer the advantage of enabling more intimate chemical interaction with the functional acid monomers. However, the problem is how these interactions can result in long-term, stable calcium-carboxylate or calcium-phosphate bonds within a hydrophilic environment where they are subject to hydrolysis.
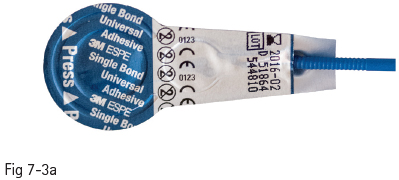
Fig 7-3 a Strong self-etch adhesive – Single Bond Universal Adhesive (3M).

Fig 7-3 b Mild self-etch adhesive – Clearfil S3 Bond (Kuraray).
Therefore, the bonding potential of the mild self-etch adhesives to enamel is weak. In order to solve these problems, self-etch adhesives have been improved by changing the pH of their primer to an intermediary value of around 1.5. This creates a more gradual transition at the dentin interface of the hybrid layer (almost complete demineralization) to the underlying unaffected dentin, reducing the long-term degradation potential. At the enamel layer, it enabled more efficient micromechanical interlocking, namely a higher bond strength (Figs 7-3a and 7-3b).
7.2.1.3 “Glass-ionomer” bonding approach
In principle, glass ionomers are the only biomaterials that self-adhere to tooth structures without any surface pretreatment. However, pretreatment with weak polyalkenoic-acid conditioner significantly improves the bonding efficiency. Consequently, the glass-ionomer approach can be achieved following a one- or two-step application procedure. The initial, surface-conditioning step becomes more important, when the tooth preparation produces more smear layer. In general, such a polyalkenoic-acid conditioner is applied for 10 to 20 seconds and rinsed off, followed by gentle air-drying without dehydrating the surface of the substrate.1,36
The increase in bonding efficiency that results can be partly attributed to a cleaning effect, by which debris is removed from the surface, to a demineralization effect, by which microporosities for micromechanical interlocking and hybridization are produced, and, above all, to the chemical interaction between polyalkenoic acid and residual hydroxyapatite. In this way, a network of hydroxyapatite-coated collagen fibrils interspersed by the micropores produced by the acid is exposed to a depth no deeper than 1 µm.
All experimental and laboratory investigations have demonstrated that this conditioner cannot be completely rinsed off; a layer up to 0.5 µm thick, referred to as a “gel phase”, remains attached to the tooth surface.
The self-adhesion of glass ionomers to tooth structures has a twofold mechanism. The first consists of micromechanical interlocking achieved by a shallow hybridization of the microporous, hydroxyapatite-coated collagen fibril network. In this respect, glass ionomers can be considered as adhering to tooth structures through a “mild” self-etch approach, with the difference being that glass ionomers are self-etching through the use of a polycarboxyl-based polymer with a relatively high molecular weight, whereas resin-based self-etch adhesives make use of monomers with a much lower molecular weight.36
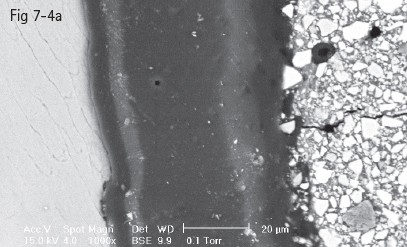
Fig 7-4 a Homogenous hybrid layer in intimate contact with enamel prisms.
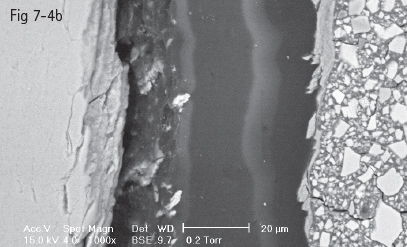
Fig 7-4 b Hybrid layer with dehiscent areas at interface with tooth substrate. (Images courtesy of Prof Dr Ion Patrascu.)
The second component of the self-adhesion mechanism concerns chemical bonds, which are ionic and formed between carboxyl groups of the polyalkenoic acid and calcium of the hydroxyapatite that remains around the exposed surface collagen. This aspect has been documented at both the enamel and dentin layers. Experiments have demonstrated that carboxyl functional groups interact with the hydroxyapatite surface. The interaction is strong enough to be recorded after ultrasonically rinsing off the polyalkenoic acid solution from the tooth substrate.2,36
The binding energy results from the oxygen and carbon atom interaction in the carboxyl group. An “adhesion-decalcification” model was proposed to explain why certain acids (such as the 10:1 acrylic/maleic acid) adhere to tooth tissue more than they decalcify it. This aspect depends upon the solubility of the formed calcium salt in its own acidic solution. The more soluble the calcium salts of the acids, the less it will adhere to the mineral substrate. As the calcium salts of the polyalkenoic acids are difficult to dissolve, they have an adequate chemical bonding potential to hydroxyapatite-based structures.
Typical for glass-ionomer adhesion is the presence of a gel phase at the interface that represents the formation of a calcium polycarboxylate salt resulting from either the polyalkenoic acid conditioner or the glass-ionomer material itself. This phase has been proved to be stable and strong, an intermediary between the shallow 0.5 to 1 µm hybrid layer and the glass-ionomer matrix. Experimental research has confirmed that this structure is more resistant to tensile forces than the glass-ionomer matrix (Figs 7-4a and 7-4b).
7.2.2 Remarks on adhesion to enamel
Buonocore demonstrated that acid etching of the enamel increased bond strength between etched enamel and composite resin.2,3,7,37 Initially, Buonocore used 85% phosphoric acid for tooth surface preparation; later, others experimented with hydrochloric, nitric, lactic, citric, pyruvic, and acrylic acid or EDTA.38–41 Several in vitro studies showed that a 35% to 37% concentration of phosphoric acid is best for acid etching of the enamel. Other studies did not document any correlation between phosphoric acid concentration and resistance to tensile force. The ideal length of time of etching is 20 to 40 seconds.2,37,41,42
Acids dissolve hydroxyapatite, the main mineral from which dental enamel is composed. Acid etching removes about 5 to 30 μm of the enamel surface.2,43,44
The amount of dissolved mineral and the depth of micromechanical retention, as well as the type of acid etching pattern, depend upon two categories of factors:
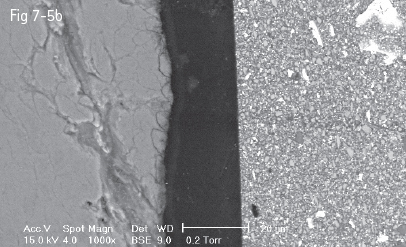
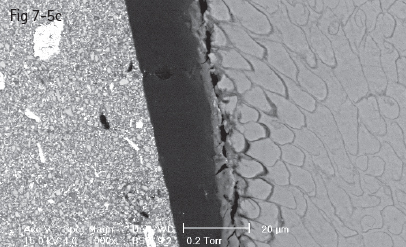
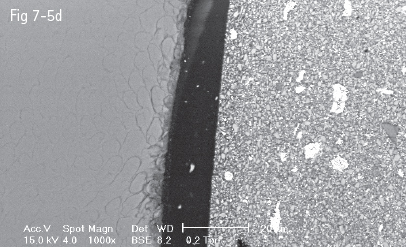
Fig 7-5 a-d Acid etching of the enamel – prisms cut longitudinally (a) and transversally (b to d) displaying the three acid etch patterns. (Images courtesy of Prof Dr Ion Patrascu.)
< div class='tao-gold-member'>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses