1960s first generation
Development of the surface-active comonomer NPG-GMA
No recommendation for dentin etching
Relied on adhesion to the smear layer through chelation
Weak bonding strength (no wetting of the dentin and did not penetrate the entire depth of the smear layer) 2–3 MPa
End of the 1970s and beginning of the 1980s second generation
Introduction of phosphate ester dentin-bonding systems
No recommendation for dentin etching
Relied on adhesion to the smear layer through chelation
Weak bonding strength (no wetting of the dentin and did not penetrate the entire depth of the smear layer) 5–6 MPa
1980s third generation
Acid etching of the dentin
Preservation of a modified smear layer with slight demineralization of the underlying intertubular dentin
Separate primer
Increased bond strength 3–8 MPa
Mixed clinical results
Early 1990s fourth generation
Three-step total-etch systems
Phosphoric acid/a primer containing reactive hydrophilic monomers in ethanol, acetone or water/unfilled or filled resin-bonding agent
Hybrid layer (resin-dentin interdiffusion zone) and penetration of open dentinal tubules, forming resin tags
Increased bond strength 13–30 MPa
Technique sensitivity
Mid-1990s fifth generation
Simplification of bonding procedure: ‘one-bottle’ system (primer and adhesive in one bottle)
Maintenance of high bond strengths 3–25 MPa
User friendlier system
Late 1990s – early 2000s sixth generation
Self-etching primer systems
Reduced incidence of postoperative sensitivity
Bond strength lower than fourth and fifth generations, except for Clearfil SE Bond
Late 2002 seventh generation
‘All-in-one’ systems
Combines etching, priming and bonding
Mild self-etching approach leads to good bond strength
Enamel etching with phosphoric acid is still recommended
End 2000s eighth generation
Still a matter of debate
5.3.1 Scientific Classification of Modern Adhesives
A more simple and straightforward way to classify adhesives is to follow the classification according to their adhesion strategy towards the enamel and dentin [18] and refer to:
-
Etch-and-rinse (E&R) adhesives (a): three-step E&Ra/3E&Ra (fourth generation) and two-step E&Ra/2E&Ra (fifth generation)
-
Self-etch (SE) adhesives (A): two-step SEA/2SEA (fifth generation) and one-step SEA/1SEA (seventh generation, …) (both 2SEA and 1SEA are further subdivided in ‘mild’ and ‘intermediately strong’ (1/2SEA-m), with a Ph ≥ 1.5, and ‘strong’ (1/2SEA-s), with a Ph < 1.5.)
-
Glass ionomers (GI) (the classification of Table 5.3 will be used in this chapter)The degree of substance exchange differs amongst these adhesives. The degree of exchange induced by etch-and-rinse adhesives exceeds that of self-etch adhesives. Today, however, systems exist with an intensive interaction with both the enamel and dentin.
In 2014, Peumans et al. [19] came to the following conclusion in a systematic review with regard to the clinical effectiveness (annual failure rate – AFR) of contemporary adhesives for the restoration of non-carious cervical lesions: the lowest AFR scores [mean (SD)] were recorded for GI [2.0 (1.4)] shortly followed by 2SEA-m [2.5 (1.5)], 3E&Ra [3.1 (2)] and 1SEA-m [3.6 (4.3)] (Tukey Contrasts: p > 0.05). Significantly higher AFR scores were recorded for 1SEA-s [5.4 (4.8)], 2E&Ra [5.8 (4.9)], and 2SEA-s [8.4 (7.9)] (p > 0.05). In addition, significant differences in AFR were noticed between adhesives of the same class (Kruskal–Wallis sum test: p > 0.05), except for GI (p = 0.7) and 2SEA m (p = 0.1).
5.3.2 ‘Smear Layer-Removing’ and ‘Smear Layer-Modifying’ Adhesives
5.3.2.1 From the Beginning Up to the Third-Generation Adhesives
Many attempts were made to ‘bond’ the adhesive to the unetched dentin substrate. Most of these, however, remained disappointing. Phosphoric acid etching of dentin was established by Fusayama et al. in 1979 [20]. This dentin acid-etching technique was discouraged in Europa and America until the end of the 1980s because of its thought pulp-irritating nature. So, most of the third-generation adhesives were still designed not to remove the entire smear layer, but rather to modify it and allow penetration of acidic monomers such as phenyl-P.
Surface-Active Comonomer NPG-GMA and the First-Generation Adhesives
NPG-GMA (N-phenylglycine glycidyl methacrylate), a surface-active comonomer, was the basis of Cervident (S.S. White, Lakewood, NJ, USA), the first commercially available dentin-bonding agent [21]. This comonomer could chelate with calcium on the tooth surface and was able to generate water-resistant chemical bonds to dentinal calcium [22, 23]. The in vitro dentin bond strengths of the first-generation dentin adhesives were in the range of 2–3 Mpa [24].
Phenyl-P (2-Methacryloxy Ethyl Phenyl Hydrogen Phosphate) and the Second-Generation Adhesives
Several phosphate ester dentin-bonding systems were introduced in the early 1980s. These products were based on phosphorous esters of methacrylate derivates. The mechanism of action was based on enhanced surface wetting and ionic interaction between negatively charged phosphate groups in the resin and positively charged calcium in the smear layer [24]. Clearfil Bond System Fc (Kuraray, Osaka, Japan) was recognised as the first product of the second generation of dentin adhesives (phenyl-P and hydroxyl methacrylate [HEMA] in ethanol). Bond strengths ranged between 1 and 6 Mpa [25–27] as a result of a lack of dentin wetting (large contact angles) [28]. Moreover, the entire depth of the smear layer was not penetrated up to the dentin to establish ionic bonding or create resin extensions into the dentinal tubules [26]. Inadequate hydrolytic stability in the oral environment was also described [29, 30].
10-MDP (10-Methacryloyloxy Decyl Dihydrogen Phosphate) and the Third-Generation Adhesives
In 1984, Clearfil New Bond (Kuraray, Osaka, Japan) was introduced and with this the concept of phosphoric acid etching of dentin before the application of a phosphate ester-type bonding agent. This phosphate-based material contained HEMA and a 10-carbon molecule known as 10-MDP, which includes long hydrophobic and short hydrophilic components [31].
Most other third-generation materials, however, were designed not to remove the entire smear layer.
4-META (4-Methacryloxyethyl Trimellitic Anhydride) and the Third-Generation Adhesives
In 1982, Nakabayashi described the hybrid layer as an interconnection zone between adhesive and dentin based on a micromechanical bonding mechanism as still used by the present-day adhesive systems [32]. Extensive research in Japan had shown that strong, long-lived bonds between resin and living dentin could be formed when a monomer such as 4-META, which contains both hydrophilic and hydrophobic chemical groups, penetrated the dentin and polymerised in situ. This resin impregnation creates the so-called transitional ‘hybrid’ layer, that is neither resin nor tooth, but a hybrid of the two. The thin layer of resin-reinforced dentin locks the two dissimilar substances together on a molecular level, sealing the surface against leakage and imparting a high degree of acid resistance. Dentin was etched with an aqueous solution of 10 % citric acid and 3 % ferric chloride. After rinsing and drying, an aqueous solution of 35 % HEMA and a self-curing adhesive resin containing 4-META, MMA (methyl methacrylate) and TBB (tri-n-butyl borane) was applied.
5.3.2.2 The Present Generations of Adhesives: Etch-and-Rinse and Self-Etch Adhesives
At present, adhesives are marketed as four different systems: three bottles or the three-step etch-and-rinse adhesives (3E&Ra); two bottles, be it an etch-and-rinse system (2E&Ra) or a two-bottle self-etch system (2SEA); and one bottle or the ‘all-in-ones’ (1SEA).
Three-Step Total-Etch Adhesives
The total-etch approach originated in Japan, where the application of a phosphate ester type of bonding followed an etch procedure with phosphoric acid [20]. Research during the begin period of the 3E&Ra did not demonstrate improvement in bond strengths, most probably as a result of the hydrophobic nature of the phosphonated resin [33]. Today, however, in vitro tensile bond strengths between 20–50 Mpa for enamel and 13–80 for dentin are demonstrated [34].
Acid etching alters the mineral content of dentin and changes its surface-free energy [35, 36]. The etched dentin (the resultant collagen network depleted of high surface energy hydroxyapatite) ends up as a low surface energy substrate. The primer in a 3E&Ra is needed to increase the critical surface tension of the dentin [34]. Both primer and bonding resins impregnate the intertubular dentin and a resin-dentin interdiffusion zone of hybrid layer is formed. Both resins also penetrate the dentinal tubules and form polymerised resin tags.
Two-Step Etch-and-Rinse Adhesives
The 2E&Ras (fifth-generation adhesives) were a simplification of the adhesive system. Most of these systems have fallen somewhat short of their objective, but still, comparable bond strengths to those of the 3E&Ra (fourth-generation adhesives) are achieved [16].
Two-Step Self-Etch Adhesives
A further demand for simplification resulted in the development of systems without a separate conditioning phase. This sixth generation consists of systems with a self-etching primer and a separate adhesive resin and those that combine the conditioner, primer and adhesive resin but require mixing and hence, the confusion when using the classification based on generations.
This group of adhesives use the smear layer on the enamel and dentin as bonding substrate as was seen with the second-generation adhesives. The acidity of the primer, however, is the main difference between the two generations. This generation adhesives contain acidic monomers such as 4-MET and 10-MDP [37, 38] which renders these adhesives much more hydrophilic compared to previous hydrophobic adhesive systems [39, 40].
The self-etching primers (SEPs) have been classified in three groups: mild, moderate and aggressive. Mild SEPs appear to provide excellent dentin bond strengths and poorer enamel bonds. Aggressive SEPs provide the reverse.
One-Step Self-Etching Adhesives
This generation of adhesives combine conditioning, priming and application of adhesive resin. Adhesives belonging to this generation are mixes of hydrophilic and hydrophobic components [41]. Furthermore, these all-in-one adhesives contain uncured ionic monomers that bind to composite restorative materials directly [42, 43].
All-in-one adhesive systems must be acidic enough to demineralise the enamel and the dentin smear layer. As a consequence, the hydrophilicity of their resin monomers is high and might result for some insusceptibility for water degradation [44]. Due to the complex nature of the mixed solutions, these adhesives are prone to phase separation and formation of water droplets within their adhesive layers [41]. The adhesive layers can also act as semipermeable membranes, permitting bidirectional water currents [45]. Lower bond strengths were registered with the all-in-one systems as compared to the 3E&Ra and the 2E&Ra [45, 46]. More recent investigations demonstrate higher bond strengths to dentin for 2SEA (Clearfil SE Bond) as compared to 1SEA. The performance of one-step self-etch systems to dentin appears to be material dependent [47, 48].
5.3.3 Recommended Approach
Today (2015), a choice can be made between the etch-and-rinse and the self-etch approach [49]. Investigations have demonstrated that micromechanical interlocking, as created by the etch-and-rinse approach, remains of paramount importance for enamel adhesion [2]. In spite of functional monomers in self-etch adhesives designed to chemically interact with hydroxyapatite, the structure, size, and orientation of enamel hydroxyapatite crystals appear to provide insufficient chemical bonding sites to achieve durable bonding to enamel. The chemical interaction of functional monomers with hydroxyapatite following a ‘mild’ self-etching approach results in an improved bond durability to dentin. Examples of these functional monomers are 10-MDP and 4-MET [50, 51].
The advocated protocol in ‘2015’ is as follows [49]:
-
Cavity margins are finished with a bevel taking into account the orientation of the enamel prisms. The ends of the enamel rods, exposed by bevelling, are more effectively etched than otherwise occurs when only the sides of the enamel rods are exposed to the acid etchant.
-
Selective etching of the enamel during 15 s with phosphoric acid (35–37 %) followed by rinsing. Etching of the dentin should be avoided, though some contact is not detrimental.
-
A 10-MDP-based mild self-etching primer is actively rubbed onto the etched enamel and the unetched dentin for 15 s minimum. The primer film is air thinned until the film no longer moves (10-MDP ionically bonds to hydroxyapatite and self-assembles into nanolayers).
-
A solvent-free adhesive resin (bonding layer) is applied, air thinned and light cured.
5.4 Erbium Laser Irradiation of the Enamel and Dentin and the Resultant Surface Changes
Lasing of the enamel and dentin results in a very specific surface morphology. Erbium laser irradiation of enamel and dentin yields an anfractuous surface (fractured and uneven) and open tubules without smear layer, both apparently ideal for adhesion [52]. Typical, lased enamel surfaces are irregular with typical keyhole-shaped prisms and rods; lased dentin shows protrusion of dentinal tubules with a cuff-like appearance (more intertubular than peritubular dentin is removed) [52–54].
The described characteristic topography of lased dentin and enamel is the result of specific ‘erbium laser-tissue’ interaction. The energy delivered by the Er:YAG (2.94 μm) and the Er,Cr:YSGG (2.78 μm) is well absorbed in the endogenous water and has a high affinity for hydroxyapatite (Chap. 4). The mechanism of enamel and dentin removal is referred to as ‘photothermal fragmentation’ or ‘ablation’.
A number of investigations have demonstrated that the presence of endogenous water is not sufficient to remove the enamel and dentin. Moreover, it has been demonstrated that Er:YAG and Er,Cr:YSGG behave differently in this respect. The small penetration depth of the Er:YAG laser generates a high spatial energy density, hence making the tissue more sensitive to interaction with the radiation. The penetration depth of the Er,Cr:YSGG laser is greater, and therefore, the corresponding spatial energy density is lower. Important to emphasise is that ablation could not be observed using the Er:YAG laser and the Er,Cr:YSGG laser in dental enamel and the Er,Cr:YSGG laser in dentin, without an external water spray [55]. Melting, cracking and carbonated hydroxyapatite mineral occurred as a result of the photothermal interaction, but also resulted in final high surface temperatures.
Er,Cr:YSGG laser radiation is stronger absorbed by the mineral of the dental enamel than Er:YAG laser radiation, but in contrast, the volumetric content of hydroxyapatite is much higher than that of water. Therefore, the absorbed radiation of an Er,Cr:YSGG laser is distributed to a large volume in the enamel, which leads to substantially lower spatial energy densities [55].
Supplying water externally makes it possible to reduce the thermal stress on the surrounding tissues and rule out damage [56, 57]. At the same time, it was demonstrated that the water spray also serves to maintain the ablation process. Different hypotheses are described and favoured, such as the formation of channels and the collapse of which causes cavitation bubbles, which in turn generate shock waves, or also so-called recoil-induced material expulsion [58]. The externally supplied water spray, or rather the water film applied by it, is the actual mediator of the ablation process and solely an absorber.
In Chap. 4, it was emphasised that a number of material characteristics and physical factors affect efficiency and quality of erbium laser ablation (Table 5.2). However, it needs to be emphasised that the role of water is very crucial. Next to endogenous water, the external water supply is of paramount importance for the ablation efficiency [55] and at the end also for the quality of the adhesion between lased tooth surfaces and adhesive restorative materials [59, 60]. Factors such as water film thickness [61] and the amount, the flow rate and the type of water spray have to be related to the energy and the pulse repetition rate [62–64].
Table 5.2
Material characteristics and physical factors affecting the efficiency and quality of Er:YAG laser ablation
|
Relevant material characteristics for biological hard tissues:
|
|
Coefficient of absorption (α)
|
|
Reflectivity of the tissue surface (R)
|
|
Specific heat capacity (cp) of the absorbing constituents in the tissue
|
|
Tissue capability for heat conduction (thermal diffusivity κ)
|
|
Distribution of water within the tissue
|
|
Properties of laser light:
|
|
Wavelength (λ)
|
|
Pulse energy (Ep)
|
|
Pulse duration (τp)
|
|
Temporal beam profile (pulse shape)
|
|
Spatial beam profile (TEM/transverse electromagnetic modes)
|
Based on this summary, it is clear that the ablation efficiency is influenced by an extensive number of parameters employed (following parameters are mostly provided in scientific publications: energy, energy density, power, frequency (Hz), beam diameter (mm), pulse duration, water flow rate, water delivery). Unfortunately, not all studies provide this information and omit important parameters (such as energy density, power, laser beam diameter, and the water delivery mode) that jeopardise inter-study comparisons [64].
5.5 Adhesion to Erbium-Lased Enamel and Dentin
5.5.1 Investigations with Etch-and-Rinse Adhesives
5.5.1.1 Enamel
De Moor and Delmé [14] concluded in their review of 2009 that acid etching with phosphoric acid remained mandatory on laser-irradiated enamel with respect to bond strength and marginal seal.
There was no value-added benefit of laser etching of the enamel after laser preparation. It has to be mentioned that laser etching is an insufficient term; laser conditioning is a more accurate term. Laser conditioning is a term used to describe a reduced ablative effect obtained with energy just above the threshold of ablation (15 J/cm2 for Er:YAG and 20 J/cm2 for Er;Cr:YSGG) (Figs. 5.1, 5.2, 5.3 and 5.4). In general, these investigations on laser etching were performed with etch-and-rinse adhesives. At that time, there was insufficient information on the effect of self-etching systems on the lased enamel.

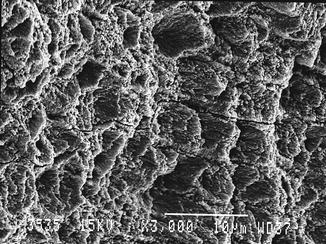
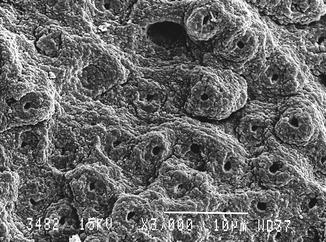
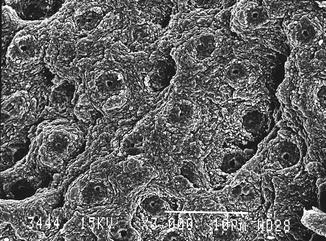

Fig. 5.1
Er:YAG laser-irradiated enamel (350 mJ, 10 Hz, 100 μs, 7 mm distance, 0.9 mm spot size) (Courtesy of Dr. Katleen Delmé and Prof. Dr. Roeland De Moor, Belgium)

Fig. 5.2
Er:YAG laser-conditioned enamel (150 mJ, 10 Hz, 100 μs, 7 mm distance, 0.9 mm spot size) (Courtesy of Dr. Katleen Delmé and Prof. Dr. Roeland De Moor, Belgium)

Fig. 5.3
Er:YAG laser-irradiated dentin (200 mJ, 10 Hz, 100 μs, 7 mm distance, 0.9 mm spot size) (Courtesy Dr. Katleen Delmé and Prof. Dr. Roeland De Moor, Belgium)

Fig. 5.4
Er:YAG laser-conditioned dentin (80 mJ, 10 Hz, 100 μs, 7 mm distance, 0.9 mm spot size) (Courtesy of Dr. Katleen Delmé and Prof. Dr. Roeland De Moor, Belgium)
5.5.1.2 Dentin
The hybrid layer created with etch-and-rinse adhesives after erbium laser irradiation of dentin and after subsequent acid etching is thinner as compared to acid-etched bur-cut dentin (Figs. 5.5, 5.6, 5.7 and 5.8).
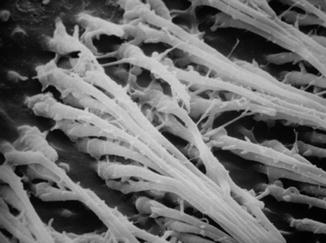


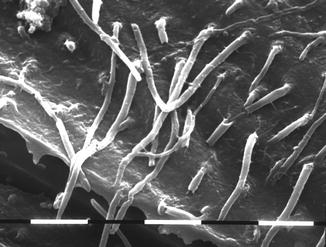

Fig. 5.5
SEM image (1,300 ×) of resin tags in the dentin (middle crown) in young third molar treated with etch-and-rinse bonding system (3M Scotchbond) (Courtesy of Prof. Luciano Fonzi and Prof. Vassilios Kaitsas, Siena, Italy)

Fig. 5.6
SEM image (1,600 ×) of resin tags in the dentin (close to border enamel-dentin junction) in young third molar treated with etch-and-rinse bonding system (3M Scotchbond) (Courtesy of Prof. Luciano Fonzi and Prof. Vassilios Kaitsas, Siena, Italy)

Fig. 5.7
SEM image of resin tags in the dentin (middle crown) using self-etch bonding system (Xeno V) after Er,Cr:YSGG preparation at 2.0 W, 20 Hz, 100 mJ, 600 μm tip, high air/water ratio (92/80 %) (Courtesy of Prof. Luciano Fonzi and Prof. Vassilios Kaitsas, Siena, Italy)

Fig. 5.8
SEM image of resin tags in dentin (middle crown) using self-etch bonding system (Xeno V) after Er,Cr:YSGG preparation at 2.0 W, 20 Hz, 100 mJ, 600 μm tip, lower air/water ratio (35/25 %) (Courtesy of Prof. Luciano Fonzi and Prof. Vassilios Kaitsas, Siena, Italy)
Different explanations have been put forward for the less profound hybridization:
-
Increase of the Ca and P because organic components are selectively removed [64].
-
Reduction of the carbon-to-phosphorus ratio leading to more stable and less acid-soluble compounds [65].
-
Formation of a modified superficial layer in which collagen fibres are poorly attached to the underlying dentin substrate [75].
-
Thermal effects also extend in the dental subsurface, impairing interdiffusion zone formation [76].
It was concluded that there is a reduced thickness of the hybrid layer after erbium laser irradiation even though phosphoric acid was used. Subsurface damage is thought to be responsible for the decrease in bond strength and cohesive failure in the subbonding layer in dentin [82–84].
Also with self-etching adhesives, both subsurface damage and the thinner or absent hybrid layer resulted in low tensile bond strengths. It was recommended in 2,009 not to use ‘non-rinse’ or ‘self-etch’ adhesives. Higher microleakage at the enamel and dentin margins was also observed [14].
5.5.2 Adhesion to the Erbium-Lased Enamel and Dentin: Investigations with Self-etch Adhesives
Most of the research from 2010 was focused on dentin adhesion. More attention was also paid to the laser irradiation mode, i.e. emphasis on low energy [87, 88], shorter pulse duration time (100 μs, but no 50 μs) [88, 90] as mentioned in 5.5.1.2 and repetition rate [85, 86]. In fact, laser treatment could enhance or impair μTBS to the enamel and dentin depending on the pulse duration used and additional acid application.
5.5.2.1 Enamel
Pulse duration of the erbium lasers is related to the laser ablation ability and surface morphology, which are a very important factor for bond strength of adhesives to both the enamel and dentin [90]. Furthermore, one study demonstrated higher microtensile bond strength (μTBS) for Er:YAG than for Er,Cr:YSGG in combination with two-step etch-and-rinse adhesives (2E&Ra) (fifth generation) [91].
In general, acid etching of enamel margins resulted in better tensile bond strengths than the sole use of two-step self-etch adhesives (2SEA) (sixth generation). Only laser-pretreated enamel surfaces appeared not to achieve clinically acceptable values due to the more acid-resistant-lased surface. These findings also coincide with the present-day recommendations still to rely on the use of phosphoric acid to etch the lased enamel margins.
5.5.2.2 Dentin
Investigations with two-step self-etch adhesives (2SEA) demonstrated that treatment of lased dentin with these 2SEA in general still resulted in lower μTBS values as compared to conventionally bur-prepared dentin surfaces [92–94]. The reduction in bond strength for Clearfil SE Bond self-etching adhesive (SEA), however, was lower than for a 2E&Ra [93]. The latter accounts for both Er:YAG and Er,Cr:YSGG [92]. These studies have in common that there is a superior adhesion for the adhesive system with 10-MDP, which has the potential to chemically interact with interfacial hydroxyapatite [93, 94].
Increasing etching time was also not able to increase the bonding strength of a 2E&Ra to irradiated dentin [92]. This is in contrast with 2E&Ra where etching during the 90 seconds resulted in increased bond strength as compared to 30 and 15 s [95]. In this respect, previous studies had already reported an increase in acid resistance of the enamel and dentin after laser irradiation [96–98]. An additional cleaning after with NaOCL after the etch procedure was also proposed [93] in order to improve the penetration of adhesive monomer into the irradiated dentin, as little or no hybrid layer formation was reported in previous studies [99, 100].
Research in the 2010s considered laser pretreatment again. Finishing the dentin at low fluency after laser cavity preparation resulted in higher bond strength then without an additional finishing for 2SEAs [101]. Depending on the formulation, a number of 1SEAs (seventh generation) demonstrate identical bond strengths on lased dentin as compared to bur-cut dentin [102]. In general, these adhesives belong to the group of ‘mild’ self-etch adhesives. More research however is needed, to explain these apparently unexpected different effects.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


