Chapter 9
Oral Mucosa and Mucosal Sensation
Ellen Eisenberg, Easwar Natarajan, and Bradley K. Formaker
Department of Oral Health and Diagnostic Sciences, School of Dental Medicine, University of Connecticut
Oral mucosa is the moist soft tissue membrane that lines the oral cavity. In the course of a single day, the oral mucosa is subject to a host of intrinsic and external environmental stimuli, irritants, and stressors, both physiologic and potentially pathological in nature. Such stimuli include the teeth, denture prostheses, foods, beverages, therapeutic and nontherapeutic foreign objects, chemical agents, extreme fluctuations in both temperature and hydration, and a vastly diverse microbial flora. Yet, despite the constant stimulation, provocation, and punishment it sustains, oral mucosa is remarkably resilient. Indeed, maintenance of oral mucosal integrity is essential for comfort, protection, tactile, thermal, and taste sensation, speech, initiation and animation of the cascade of reflexes involved in the process of preparing foods and liquids for swallowing and digestion, and reflexes, like gagging, associated with the elimination of noxious substances. Among the mechanisms that ensure preservation and optimal function of the oral mucous membrane as a whole are (1) the microstructure of the oral surface epithelium; (2) the intricate apparatus that attaches the naturally avascular epithelium to the underlying stromal tissues that provide it with critical vascular, neural, nutritional, and immunological support; (3) the mechanical strength provided by a supportive extracellular matrix; and (4) secretions from both the major salivary glands and, in particular, a multitude of submucosal minor salivary glands that serve to lubricate the oral surface, contribute to the digestive process, facilitate speech and taste sensation, and take part in various other functions essential to homeostasis.
The oral cavity – anatomy
The oral cavity is the gateway to the alimentary canal and a portal to the upper respiratory tract, and the lips mark the entry way into the oral cavity. The lips are comprised of three anatomic subdivisions, two of them external and one internal. The external or “dry” subdivisions include the skin of the lips, with dermal appendages, and the highly vascular vermilion (“red”) borders of the lips which, in contrast, lack dermal appendages (Figures 9.1a, 9.1b, 9.2). The vermilion border is sharply demarcated from the skin of the lips. Its surface is characterized by skin markings and uniform coloration (Figure 9.1a). The internal “wet” portion of the lip, the labial mucosa, demonstrates prominent vascular markings. A rich complement of submucosal minor salivary glands provides the labial mucosa with secretions that are expressed onto the mucosal surface. The secretions lubricate the soft tissues and teeth and provide comfort and protection (Figure 9.1b).
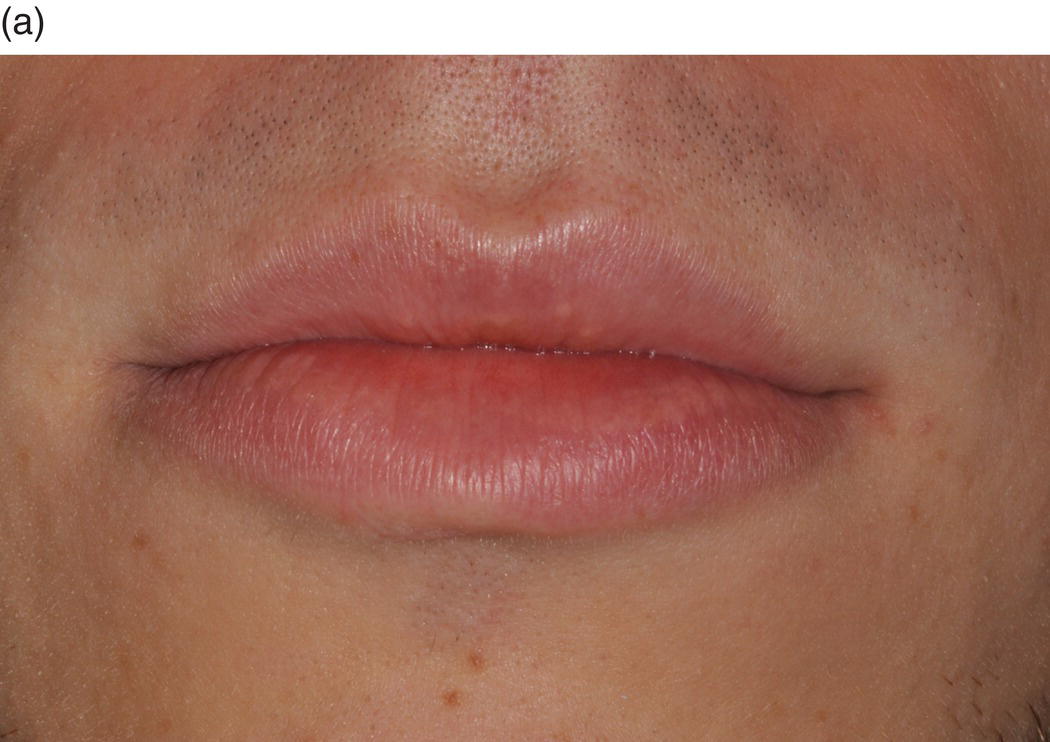
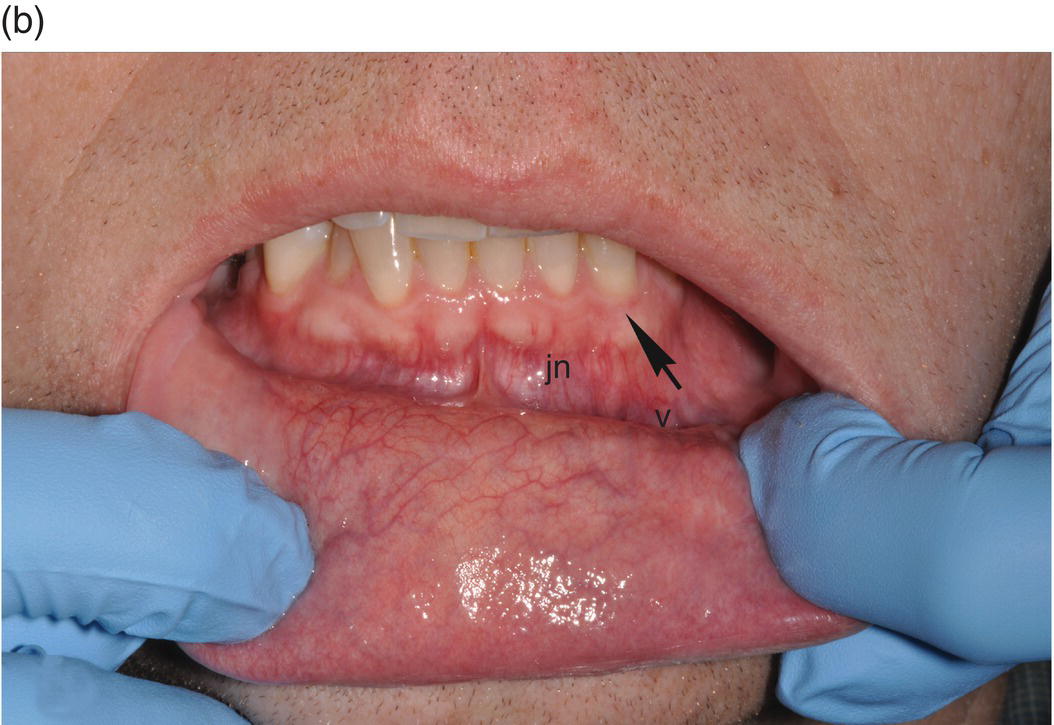
Figure 9.1 Parts of the lip. (a) External segments of the upper and lower lips. Note that the skin of the lips has hair shafts and other skin appendages. In contrast, the vermilion borders are evenly -colored, hairless, sharply demarcated from the adjacent skin, and demonstrate subtle vertical skin markings. (b) The labial mucosa is the moist internal portion of the lip. Underlying vasculature is readily discernible on the labial mucosa and the contiguous vestibular mucosa (v) and the alveolar mucosa. The junction between the pale-appearing attached gingiva (arrow) and the alveolar mucosa is the mucogingival junction (jn).
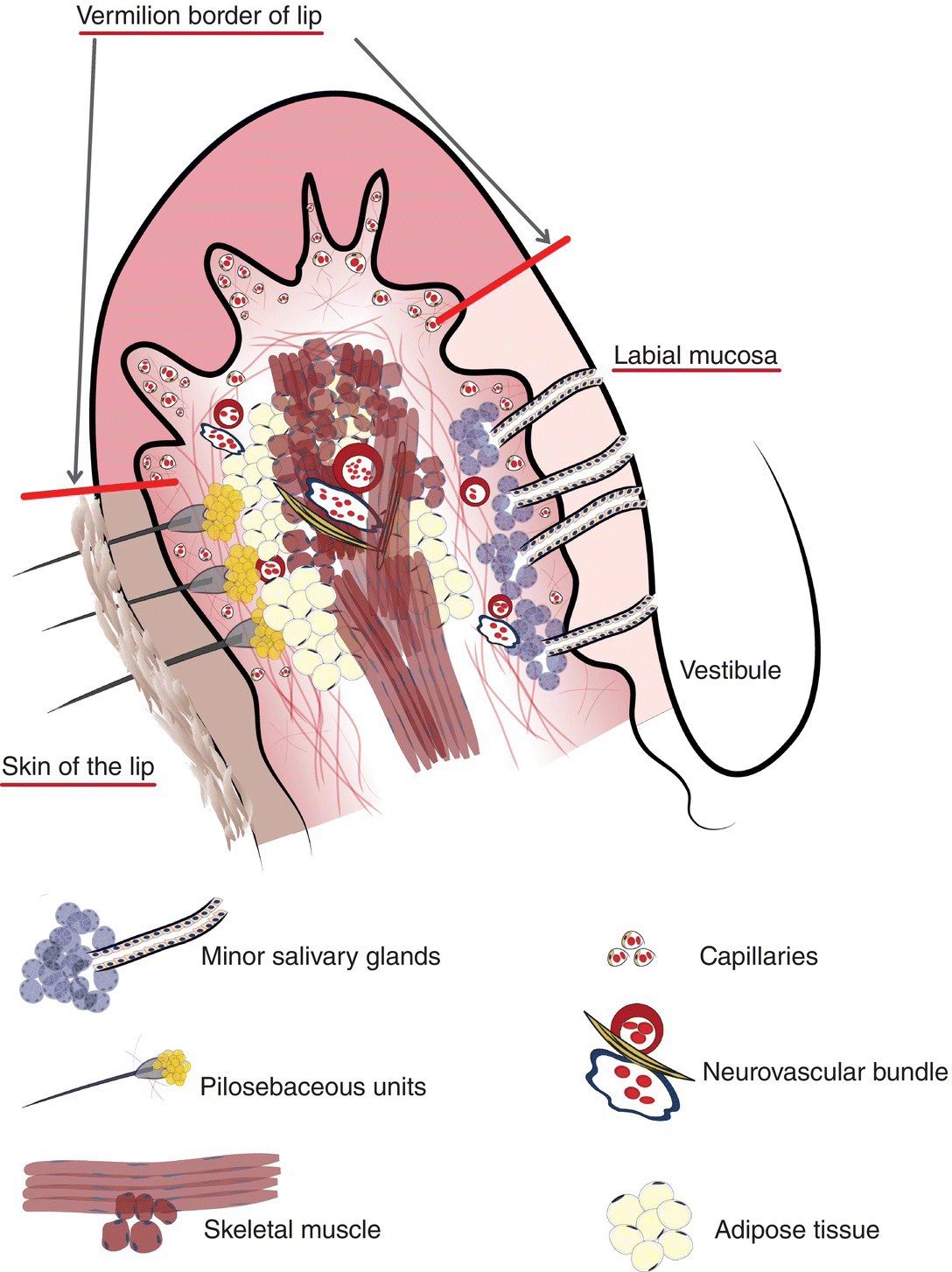
Figure 9.2 Clinical-histological representation, parts of the lip.
The external portions of the lips are exposed to extrinsic environmental stimuli and irritants, particularly fluctuations in wetting and drying and traumatic injury, especially from the teeth. In comparison to the upper lip, which resides in the shadow of the nose, the external portion of the lower lip is in a more prominent anatomic position. This is significant because over time, chronic exposure of the vermilion border and skin of the lower lip to ultraviolet light places it at particular risk for actinic (sun-related) damage. Chronic actinic-related alterations include collagen degeneration, atrophy of the epithelial surface, precancerous epithelial changes, and squamous cell carcinoma (lip cancer).
As a discrete anatomic region, the oral cavity begins where the oral mucosa begins, that is, at the junction of the vermilion border and the labial mucosa. It extends posteriorly to the palatoglossal folds, the more anterior of two arch-like soft tissue structures that mark the entrance to the oropharynx. The more posteriorly positioned of these arches are the palatopharyngeal folds at the opening of the oropharynx. The palatoglossal and palatopharyngeal folds together form the tonsillar pillars. On clinical examination, nodular, lobulated lymphoid tissue masses, the palatine tonsils, can be seen bilaterally in the tonsillar fauces, the concave tissue recesses between the palatoglossal and the palatopharyngeal folds (Figure 9.3). Along with the palatine tonsils, other smaller aggregates of oral mucosa-associated lymphoid tissues distributed in the posterior oral cavity comprise Waldeyer’s ring. They include the lingual tonsils on the posterior dorsal-lateral surfaces of the tongue, and small, raised, orange-pink colored papules on the soft palate, the posterior pharyngeal wall, and occasionally the floor of the mouth (Figure 9.4).
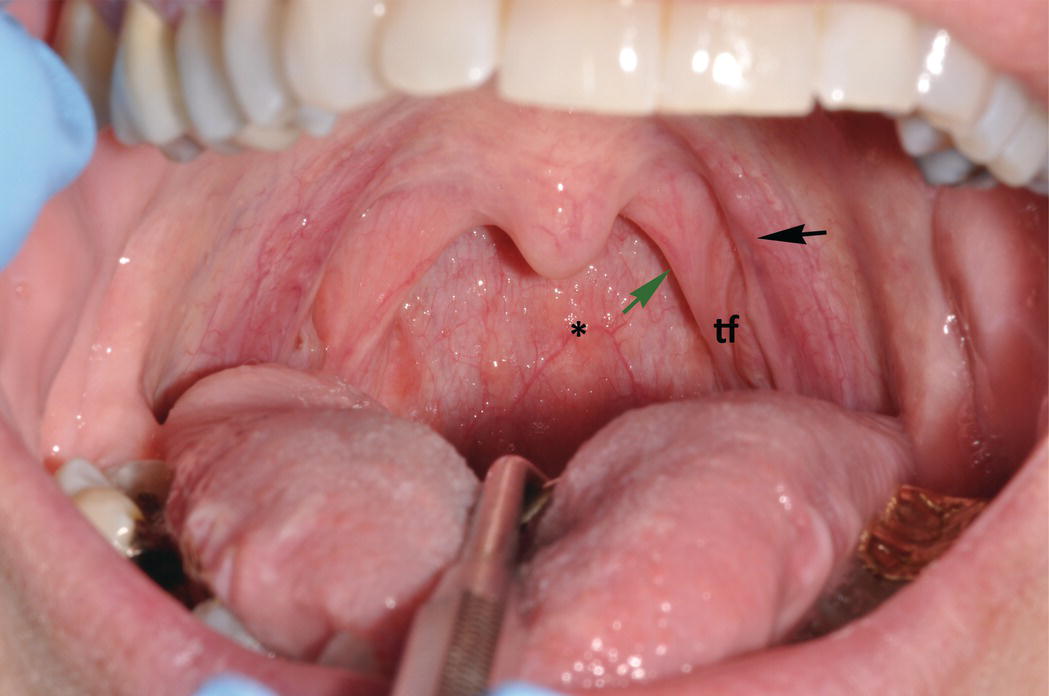
Figure 9.3 Soft palate, uvula, and palatoglossal folds. Clinical photograph demonstrates the bilateral, arch-like palatoglossal folds (anteriorly) (black arrow) and the palatopharyngeal folds (more posteriorly) (green arrow). In the depression in between these arches is the tonsillar fauces (tf) where the relatively large palatine tonsils, a component of Waldeyer’s ring of lymphoid tissue (not visible in this photograph), reside. The uvula projects down ward from the soft palate and is seen in the midline. The posterior wall of the pharynx contains several small orange-pink aggregates of lymphoid tissue (*).
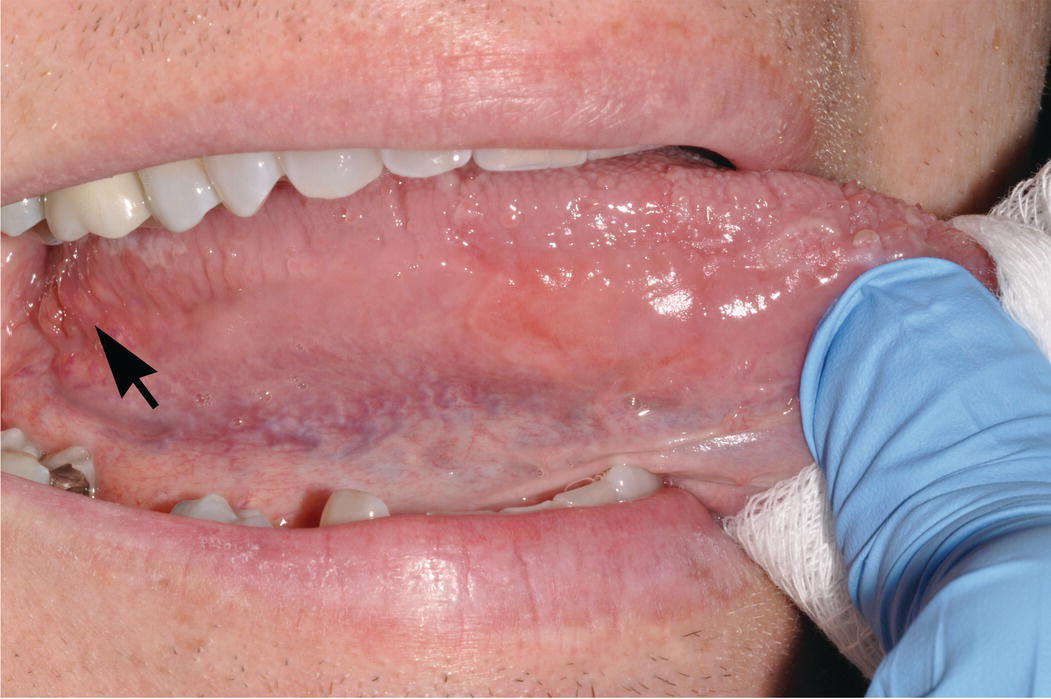
Figure 9.4 Lateral-ventral surfaces of tongue, foliate papillae. The lateral-ventral tongue surfaces are nonkeratinized. Note the transition from the relatively thick, rough and heavily keratinized dorsal surface superiorly to the smoother lateral and ventral surfaces of the tongue, inferiorly. The lingual veins are readily seen through the nonkeratinzed ventral tongue surface. Lingual tonsils (lymphoid tissue of Waldeyer’s ring) and foliate papillae (arrow) reside on the most posterior lateral-ventral surface of the tongue. The lingual tonsils are small, orange-pink nodules and lie posterior and dorsal to the foliate papillae.
The maxillary and mandibular alveolar ridges and teeth subdivide the oral cavity internally into its two major regional components, the outer of which is termed the oral vestibule; the inner portion is the oral cavity proper. The oral vestibule is bounded by the buccal mucosa and labial mucosa (inner cheeks and lips, respectively) (Figure 9.5). The deep troughs that mark the inferior and superior limits of the buccal and labial mucosae are the maxillary and mandibular vestibules. Both vestibules reflect onto and are contiguous with the alveolar mucosa that overlies the upper and lower alveolar ridges. The mucosal tissue directly below the crowns of the mandibular teeth, above the crowns of the maxillary teeth and bound down to their respective underlying alveolar bone is the attached gingiva (“gum”). The clinically evident boundary that distinguishes the flexible, thin alveolar mucosa with its visibly redder coloration from the immobile, more pale or pink stippled-appearing attached gingiva is the mucogingival junction.
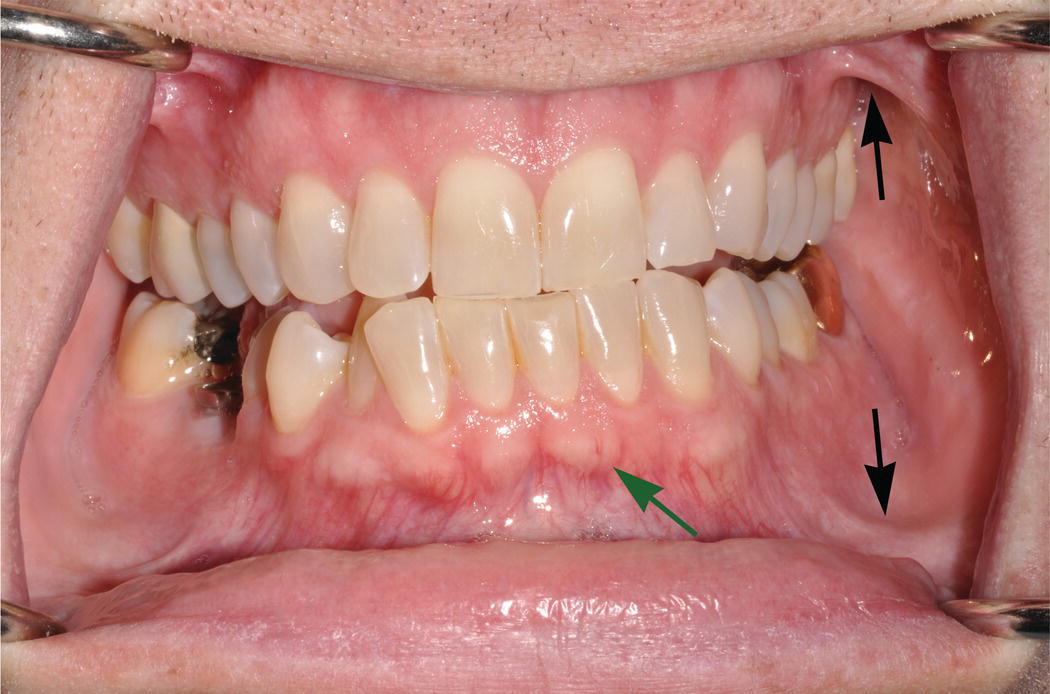
Figure 9.5 Oral vestibule. The maxillary and mandibular vestibules (troughs) are the superior and inferior boundaries, respectively, of the buccal and labial mucosae. Note the arc-like connective tissue attachments (frena or frenula) (black arrows) that traverse the vestibules and link the buccal and labial mucosae with the alveolar mucosae. Because of their thinner, nonkeratinized epithelial surfaces, underlying vasculature is visible in the vestibular and alveolar mucosae, in contrast to the denser, keratinized mucosa of the pale-pink attached gingiva. As also seen in Fig. 9.1, the mucogingival junction (green arrow) is the boundary that separates the attached gingiva from the alveolar mucosa.
The oral cavity proper is bounded by the hard and soft palates superiorly, the palatoglossal folds posteriorly, the maxillary and mandibular alveolar processes laterally and anteriorly, and the floor of the mouth inferiorly. The oral portion (or body) of the tongue projects upward and forward into the oral cavity from the floor of the mouth. It comprises the anterior two-thirds of the tongue, which ends at the terminal sulcus, a V-shaped groove on the dorsal tongue surface just posterior to and parallel with the circumvallate papillae (Figure 9.6). At the apical convergence and most posterior point of the V of the terminal sulcus is the foramen cecum, which lies immediately anterior to the base of the tongue. The base of the tongue marks the entrance to the oropharynx.
The oral cavity as a whole is indeed a distinct anatomic region of the body, and should be regarded separately from the skin and vermilion borders of the external lips and the oropharynx. Keeping this in mind, students and clinicians should be aware that epidemiological and statistical data concerning various pathological conditions of the vermilion borders and skin of the external lips, the oral cavity, and the oropharynx are frequently pooled together and reported as a single statistic. However, it is improper and potentially misleading to combine information concerning these three disparate anatomic regions. Presenting such aggregated data as equally representative of these disparate regions collectively can and has resulted in serious misconceptions concerning their individual vulnerability to various disease processes. Combining data can and has led to the erroneous impression that there are no differences among the external lips, the oral cavity, and the oropharynx relative to various tissue responses and specific disease susceptibility and experience. However, the oral cavity and its lining tissue, the oral mucosa, are indeed unique and deserve consideration independent and distinct from their nearest neighbors, the external lips anteriorly and the oropharynx posteriorly.
Oral mucosa
The general structure of oral mucosa
The entire oral mucosal surface is lined by stratified (layered) squamous (scale-like) epithelium, an avascular, highly organized, and semipermeable ectodermal tissue that varies in thickness and surface keratinization according to its location in the mouth and the functional demands of that location. Oral stratified squamous epithelium replenishes itself relatively frequently. The roughly 14 to 21 days of epithelial turnover time is necessarily rapid because of the considerable challenges oral mucosa is subjected to, functional and otherwise, throughout the waking hours and during sleep (Figures 9.7, 9.8).
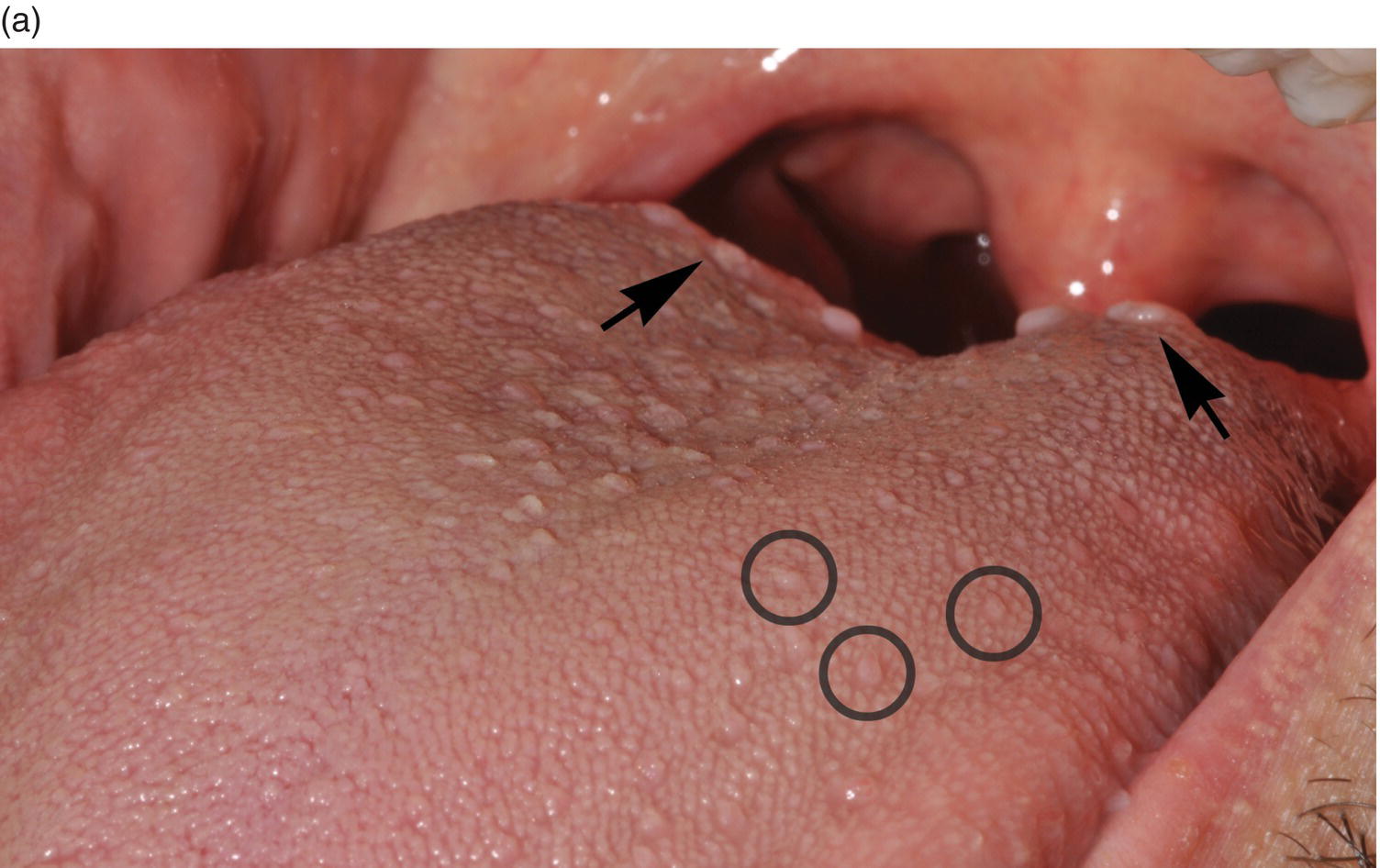
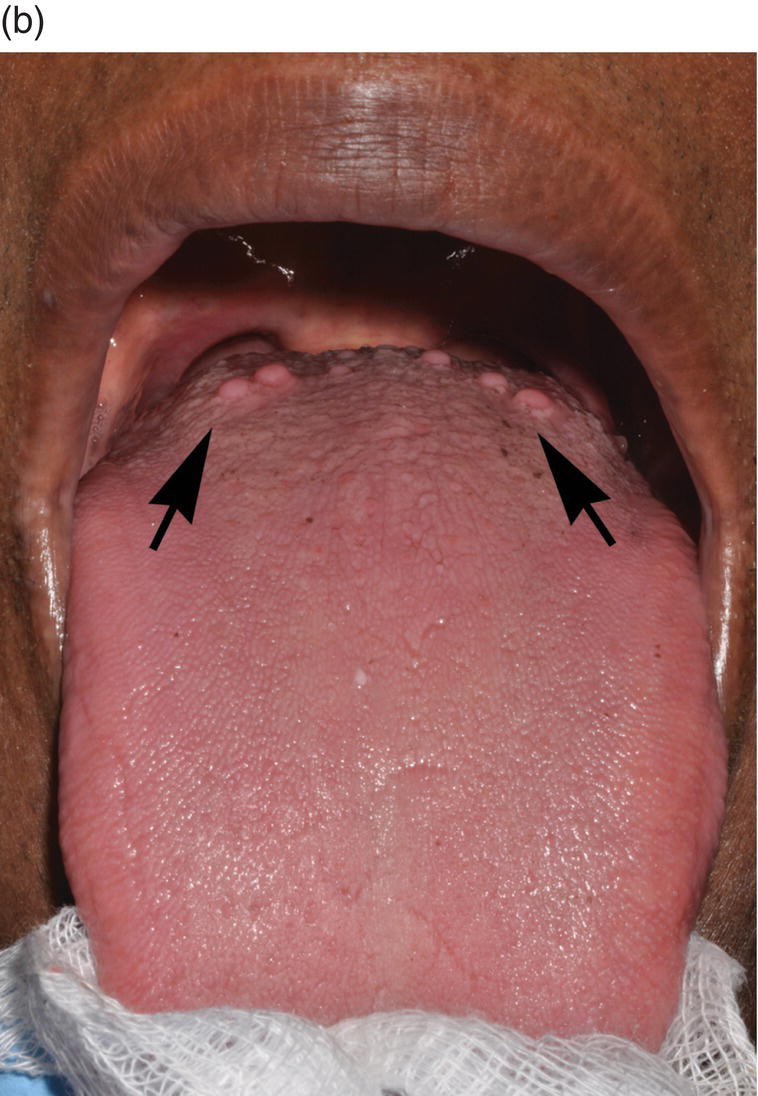
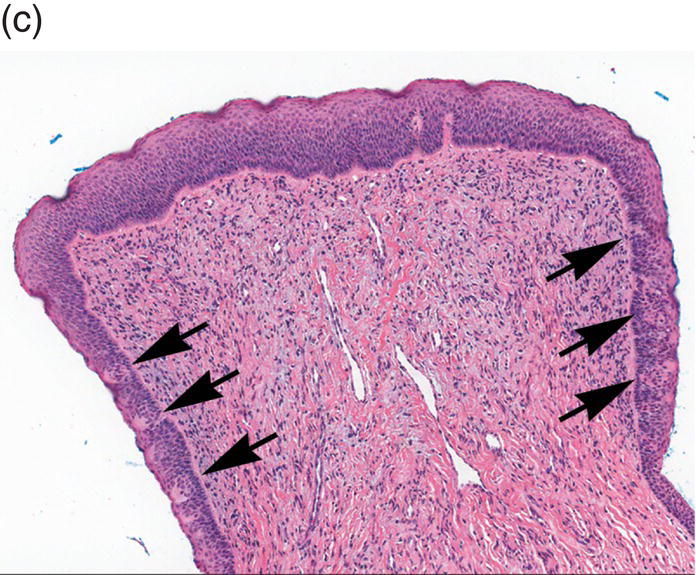
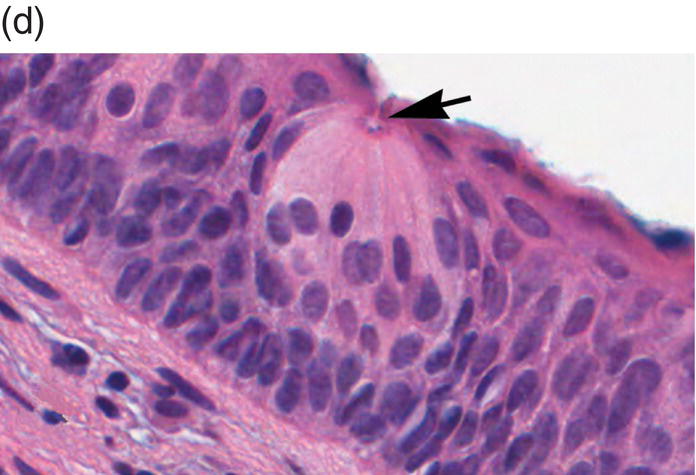
Figure 9.6 Dorsal tongue. (a) The dorsal surface of the tongue is covered by specialized mucosa. The roughness of the surface is attributable to the abundant, small hair-like filiform papillae that cover much of the anterior two- thirds of the tongue, and lack taste buds. The less numerous, small, round, white-red, papular fungiform papillae are distributed over the dorsal surface (center of grey circles). On the most posterior one third of the oral portion of the tongue are the 8 to 12 large circumvallate papillae (arrows) that are lined up in a V-formation and converge at the foramen cecum. (b) This view of the dorsal tongue demonstrates the arrangement of circumvallate papillae in a V-shaped configuration at the junction of the anterior two-thirds and the posterior one-third (arrows). (c) Taste buds (arrows) are present in the epithelium of the lateral surfaces of the circumvallate papilla. (d) An individual taste bud within the epithelium of the papillary trough. The orifice (taste pore) (arrow) of the taste bud opens into the lateral wall of the circumvallate papilla, allowing for taste sensation to be received by the taste bud.
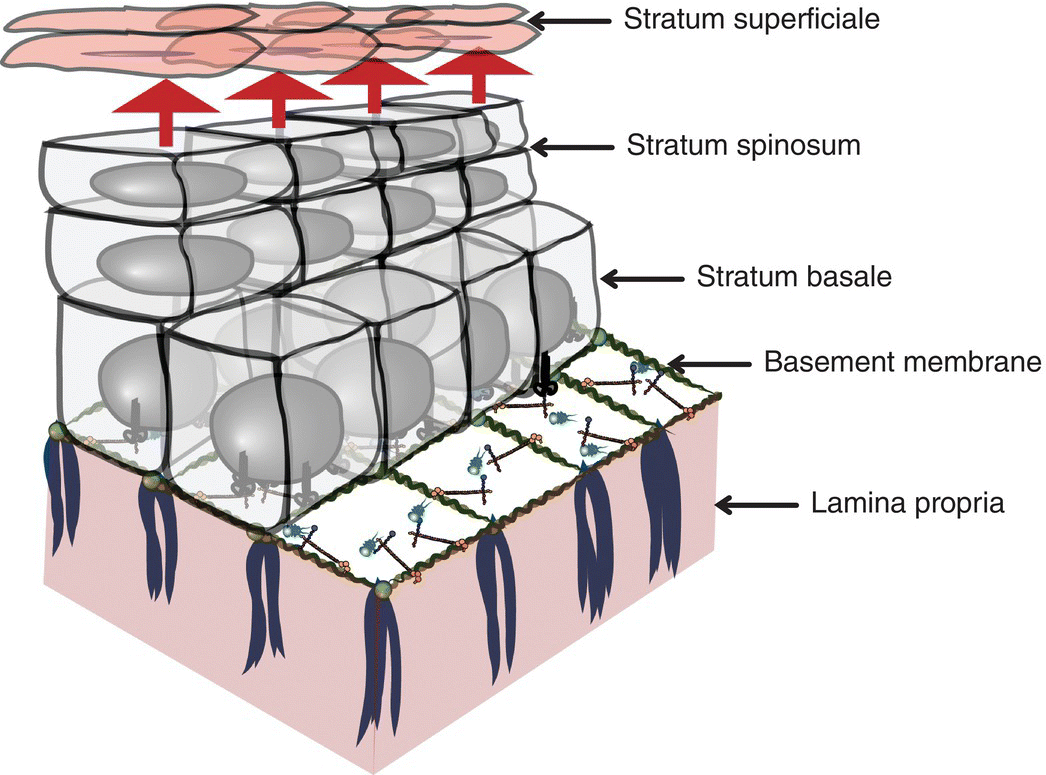
Figure 9.7 Representation of stratified squamous epithelium. Demonstration of the layers of stratified squamous epithelium as they proceed to maturation and desquamation at their apical domain. The epithelium resides on a basement membrane which overlies the lamina propria.
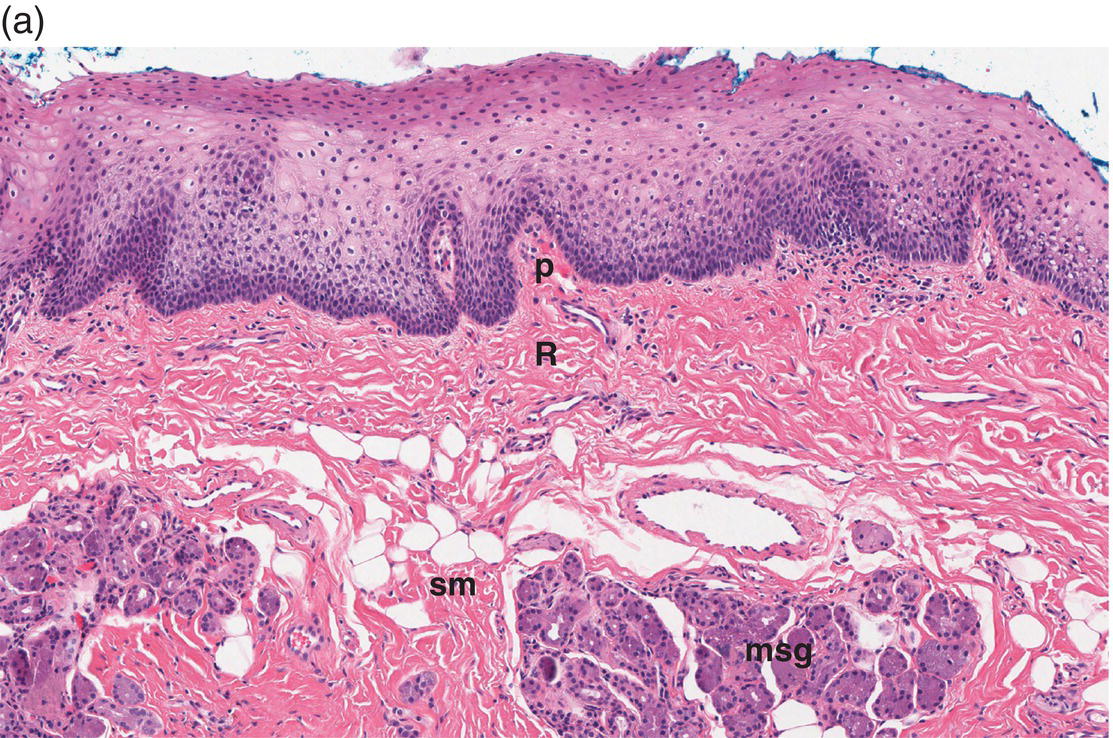
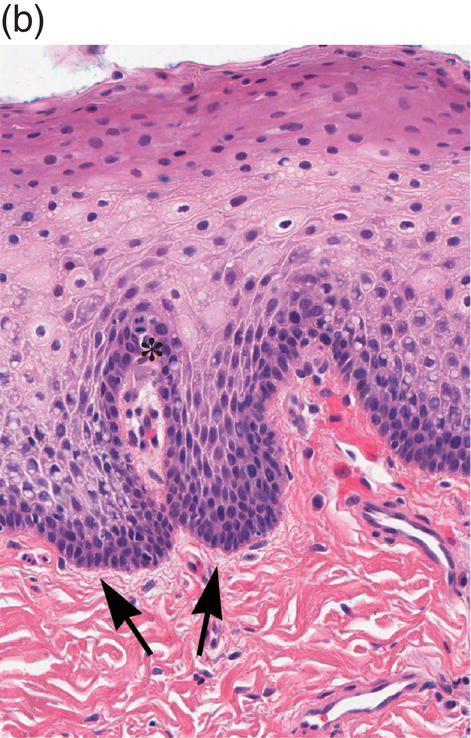
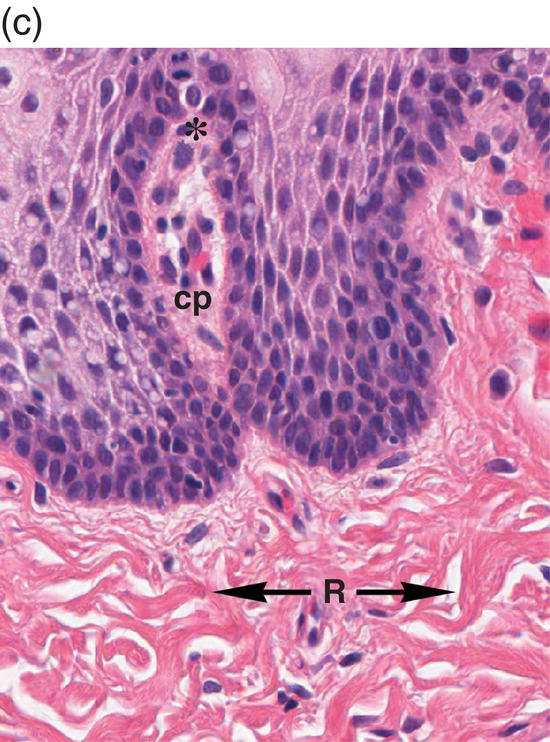
Figure 9.8 Oral mucosa. (a) At low power magnification stratified squamous epithelium overlies the papillary (p) and reticular (R) lamina propria and submucosa (sm), with adipose tissue and lobules of minor salivary glands (msg). (b) Downward, undulating epithelial rete pegs (arrows) interdigitate with the apical (upper most) (*) aspect of the papillary lamina propria. (c) Note the regimented-appearing epithelial basal cells. Stem cells reside within the basal layer at the apex of the papillary lamina propria (*). Small blood vessels (cp) and delicate collagen fibers are present in the papillary lamina propria, as contrasted with the thicker collagen fibers of the reticular lamina propria (R with arrows) that run parallel to the epithelial surface.
The basal-most portion of stratified squamous epithelium is arranged in undulating projections termed rete pegs. The rete pegs are part of the mechanism of attachment of the squamous epithelium to the basement membrane, which separates it from several layers of underlying stromal connective tissue. The most superior and widest layer is the lamina propria (Figures 9.8a, 9.8b). The lamina propria in turn is subdivided into two layers, the papillary layer and the reticular layer (Figures 9.8b, 9.8c). In some locations within the oral cavity the transition between these two layers may be subtle, making their distinction from one another difficult to recognize. The relatively loose papillary layer of the lamina propria is the more superficial segment, which lies immediately inferior to the epithelium. Here a thin, delicate fibrocollagenous tissue stroma contains vascular channels, fibroblasts, elastic fibers, and peripheral nerves. Some unmyelinated axonal endings of the stromal neural elements project upward and penetrate the overlying epithelium to function as sensory receptors, while others terminate in Meissner’s corpuscles in the lamina propria (see ”Oral Sensation” section). Additionally, efferent autonomic nerve fibers innervate blood vessels and minor salivary glands located in the lamina propria and submucosa.
Underlying the papillary layer is the reticular layer of the lamina propria (Figures 9.8a, 9.8b). The collagen bundles of the reticular layer are generally more concentrated and denser than the more slender and loose collagen fibers of the papillary layer. The reticular layer’s collagen and elastic fibers are woven together in a lattice-like network or reticulum. With the exception of the attached gingiva and the anterior portion of the hard palate and raphe, which are fixed directly to the underlying alveolar bone as a mucoperiosteum, in all regions of the oral cavity there is a submucosal layer beneath the lamina propria. The submucosal layer consists of broad bands of fibrocollagenous and elastic tissue containing blood vessels and nerves. Depending on its specific anatomic location in the oral cavity, the submucosa may also contain minor salivary gland lobules (Figures 9.8, 9.9) whose excretory ducts are contiguous with and exit onto the mucosal surface (Figure 9.2) (labial mucosa, buccal mucosa, hard and soft palates), lymphoid tissue (posterior-lateral tongue, soft palate and uvula, floor of the mouth), skeletal muscle (tongue, lips, buccal mucosa, soft palate, floor of mouth) (Figure 9.10a) and varying amounts of adipose tissue (Figure 9.9), of which there is an abundance in the buccal mucosa and soft palate. In the buccal mucosa and the vermilion borders of the lips there also may be ectopic sebaceous glands (Fordyce granules, or Fordyce spots) (Figure 9.10b).
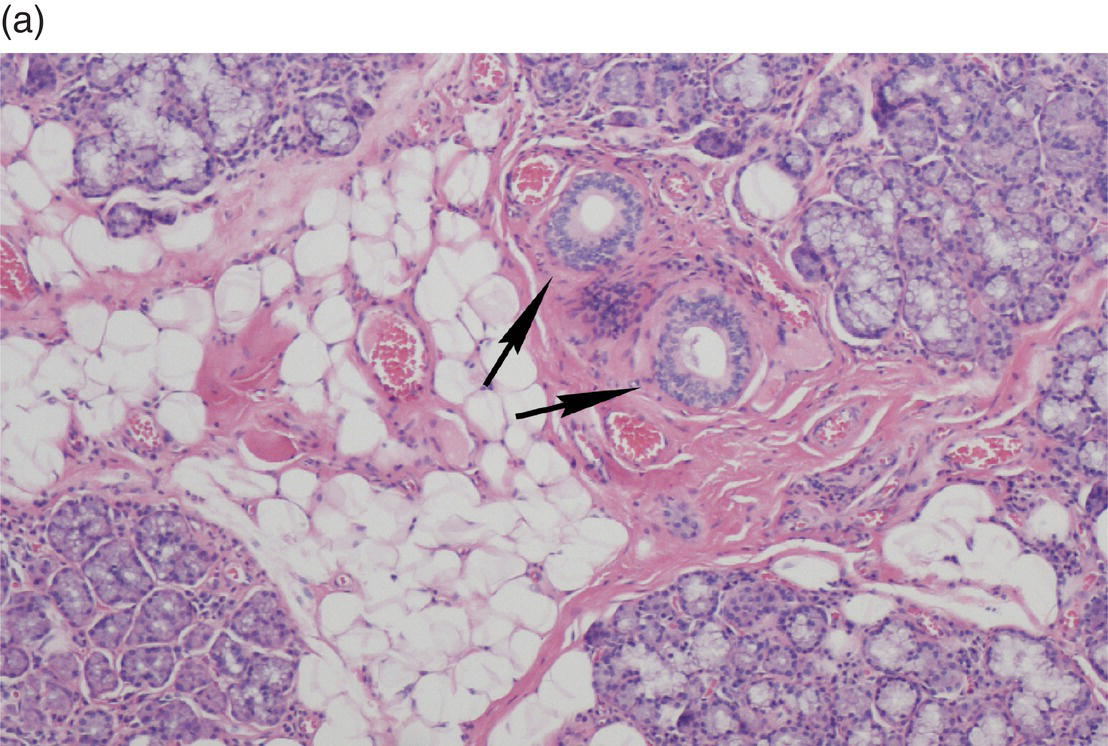
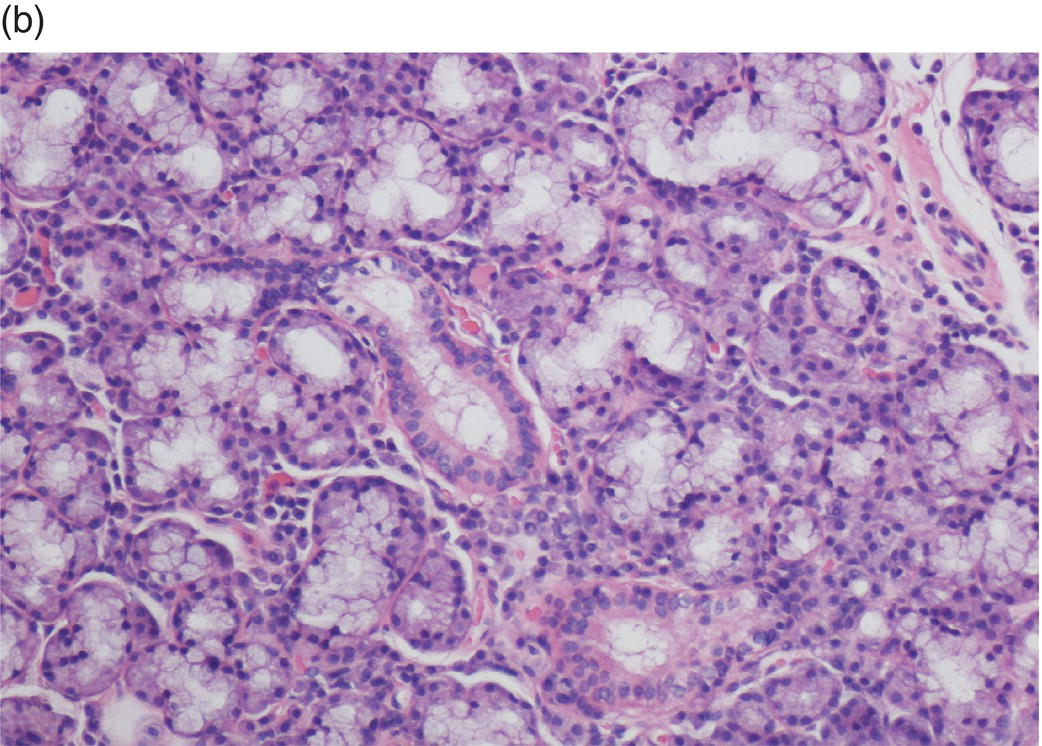
Figure 9.9 Submucosa. (a) Submucosal adipose tissue, minor salivary glands and blood vessels. Two large excretory ducts are seen centrally (arrows). (b) Minor salivary gland lobules. Mucous acini with serous demilunes, and intralobular ducts (d).
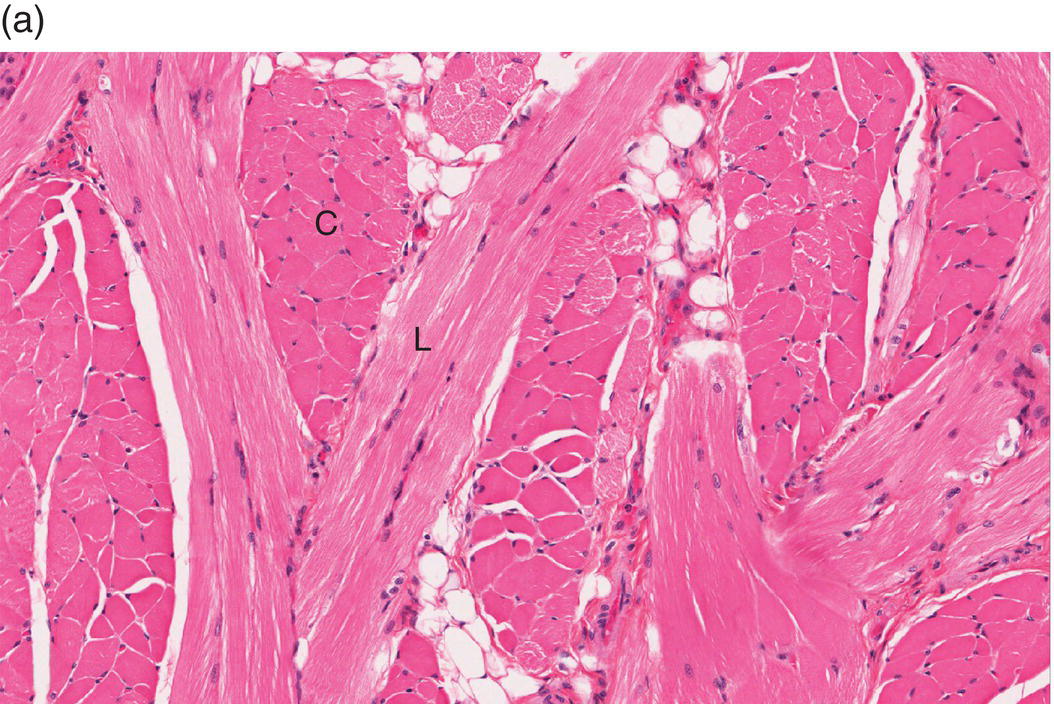
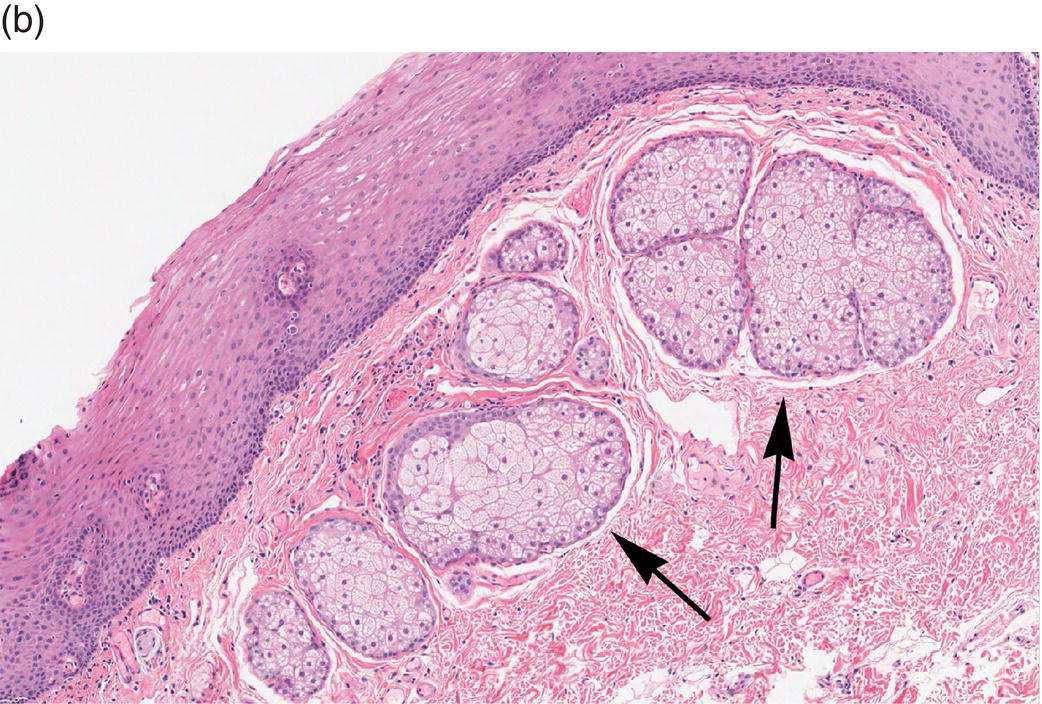
Figure 9.10 Skeletal muscle and Fordyce granules. (a) Skeletal muscle bundles, tongue. Fibers oriented in different directions are cut in longitudinal (L) and cross-section (C). Note the intervening adipose tissue. (b) Buccal mucosa, nonkeratinized epithelium. Ectopic sebaceous glands in the lamina propria (Fordyce granules) (arrows).
Stratified squamous epithelium – general principles and differentiation
Epithelium – general principles
Epithelium is the tissue that invests most external and internal body surfaces (e.g., skin, GI tract, respiratory tract, urinary tract). Also, specialized epithelial tissue is central to the function of several organs. In addition to its critical role in providing a protective barrier (skin, mucosa), specialized epithelial cells play a role in exocrine secretory function (e.g., mucous glands, exocrine pancreas), transport (e.g., respiratory epithelial cilia), filtration (e.g., renal podocytes), metabolism (e.g., hepatocytes) and thermal regulation (e.g., sweat glands). Some epithelial cells function expressly as special sensory receptors (smell, taste, vision, hearing, etc.). Regardless of the type of epithelium, they all share the fundamental characteristics enumerated below. Epithelial cells:
- reside in close apposition and adhere to one another via adhesion molecules that create cell junctions
- exhibit polarity, both in terms of function and form. Various functions correlate with morphologic surface domains (a free surface or apical domain; a basal domain; and a lateral domain) that are dictated by membrane proteins. The classification of various epithelia is based on the specific specialization or morphology of the apical (uppermost) domain
- attach to an underlying protein and polysaccharide-rich, non-cellular basement membrane at their basal-most surface.
Stratified squamous epithelium – strata, features, and differentiation
Stratified squamous epithelium invests the oral mucosa. It conforms to all of the general principles described above and is defined by the presence of squamous (scaly) epithelial cells at its apical portion and a basal domain bound down to a basement membrane. At the base, the epithelial–connective tissue interface is an undulating arrangement of epithelial projections, the rete pegs that dovetail with the connective tissue papillae of the superficial lamina propria. Similar to their counterparts in the epidermis of the skin, keratinocytes of oral mucosal epithelium are arranged in layers of cells that progress from the basal-most inferior layer upward to the surface where they assume a flattened, squamoid (scaly) morphology (Figure 9.7). Squamous epithelial cells are keratinocytes. As is characteristic of most epithelial cells, squamous cells contain predominantly an abundance of cytokeratins, intermediate filament proteins of differing molecular weights; this is in addition to actin and other filamentous proteins. The constellation of specific cytokeratins particular to each keratinocyte’s maturational stage is responsible for that cell’s individual shape and structure and its adhesion to neighboring keratinocytes. The whole program that dictates the squamoid morphology of apical cells is initiated in the basal layer.
Stratum basale/stratum germinativum/basal layer
The basal-most cell layer, the stratum basale (basal layer, stratum germinativum), consists of a single layer of basal keratinocytes (epithelial basal cells). Morphologically, basal cell nuclei tend to be round, basophilic, and large (relative to their cytoplasmic volume); the cytoplasm is cuboidal and basophilic (~10–12 μm) (Figures 9.8, 9.11). The basal cells rest on a basement membrane, the interface zone between the epithelium and the underlying connective tissue. Individual basal cells are attached to the basement membrane by hemidesmosomes. Hemidesmosomes are the defining transmembrane linkers or “integrators” of stratified squamous epithelium (Figure 9.12). They are composed of an intricate complex of proteins that are centered around a transmembrane heterodimer, specifically integrin α6β4. Within the cytoplasm the α6β4 integrin is connected to bundles of intermediate filaments and actin via intracellular linking proteins, bullous pemphigoid antigens (BP230 and BP180). The extracellular domain binds to laminin 5 (a ligand for several integrins), a glycoprotein that is one of several extracellular matrix proteins that make up the basement membrane.
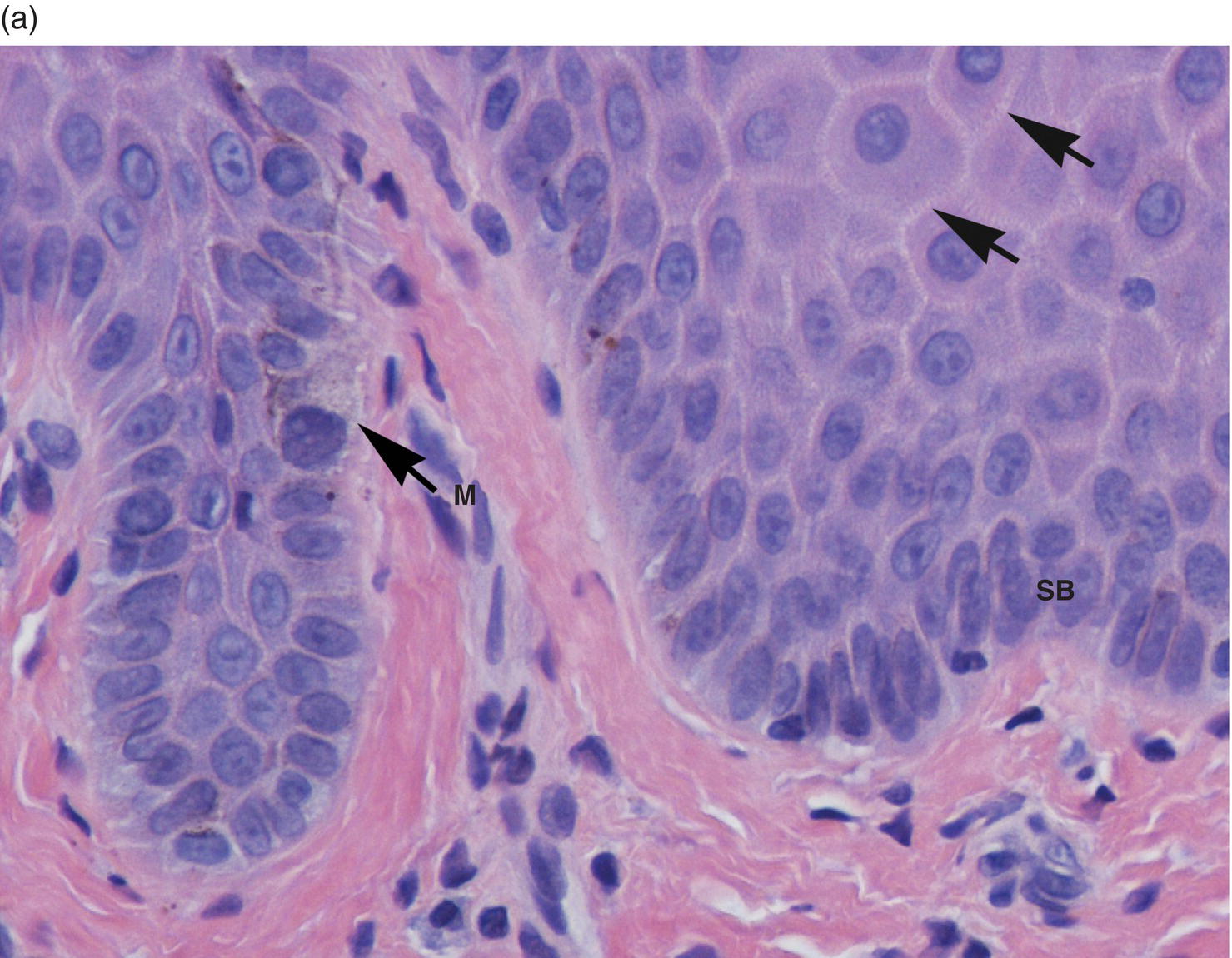
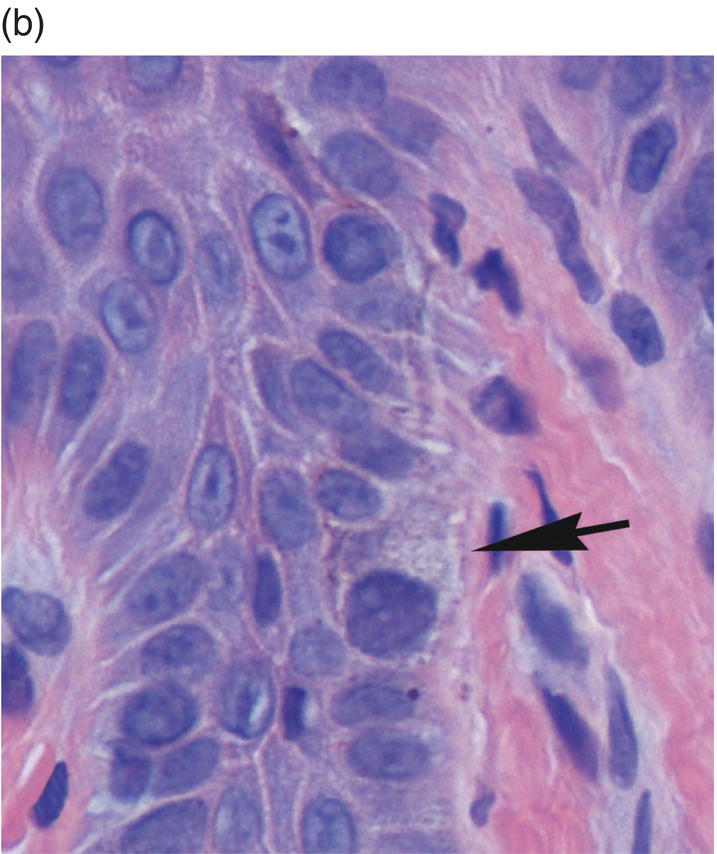
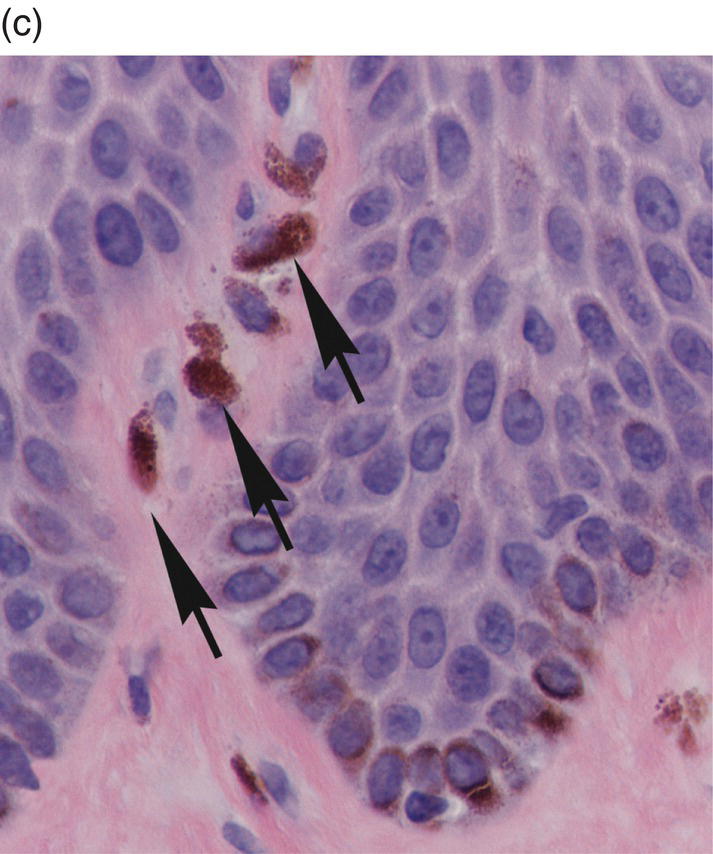
Figure 9.11 Stratified squamous epithelium; rete pegs, melanocytes, and melanin incontinence. (a) Rete pegs. The cells of the stratum basale (SB) are cuboidal and regimented with relatively large nuclei as compared to the spinous cells. Note the intercellular bridges (spines) between the polyhedral-appearing spinous cells (arrows). Among the cells of the stratum basale, a melanocyte with its dendritic projections and pigment is seen (M with arrow). (b) Melanocyte within the basal cell layer (arrow). (c) Melanin incontinence. In some chronic inflammatory processes, melanin (brown granular pigment) which is normally transported into adjacent basal cells may be spilled into the lamina propria where it is engulfed by macrophages (“melanophages”) (arrows).
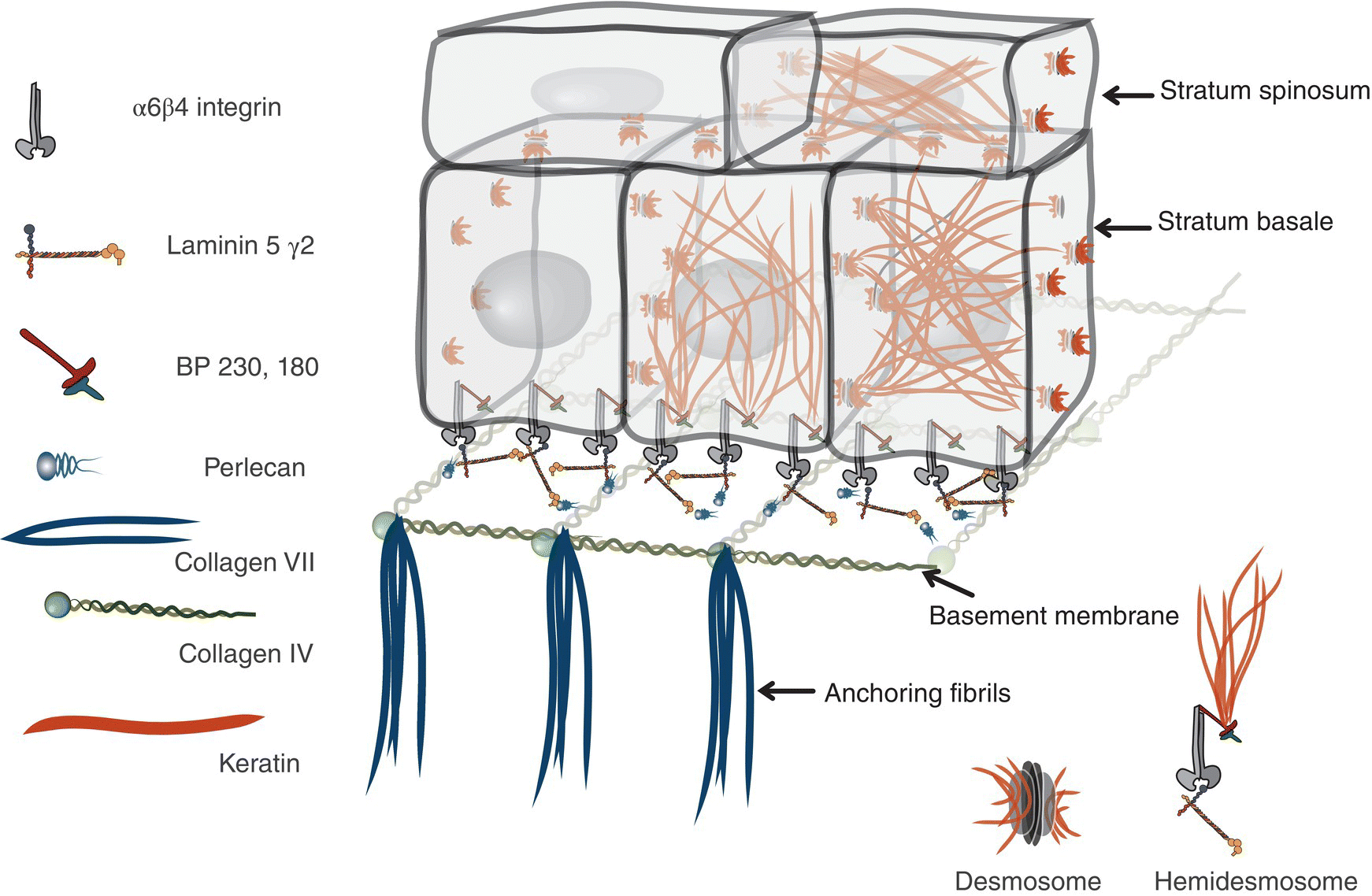
Figure 9.12 Ultrastructure of epithelial-basement membrane arrangement. Epithelial basal cells rest on a basement membrane. The basement membrane is comprised of a complex of collagen IV, Laminins, and a meshwork of various other extracellular matrix proteins (perlecan, nidogen, entactin, etc.). Anchoring fibrils attach the basement membrane to the lamina propria. Basal cells are bound to Laminin 5 via hemidesmosomes that are anchored intracellularly to filamentous proteins in the cytoplasm. Epithelial cells are attached to one another via desmosomes. They are attachment complexes comprised of placodal proteins that link with the keratin cage within the cytoplasm.
The defining characteristic of basal cells, in addition to their morphology, is their proliferative capacity (thus the alternative designation, stratum germinativum). Keratinocytes originate in the basal-most layer from a population of stem cells that undergo mitotic division. Stem cells are generally concentrated in the basal regions around the apices of the papillary lamina propria (Figures 9.8b, 9.8c). This population of stem cells undergoes division, with one daughter cell remaining behind in this region. The other daughter cell moves sideways and inferiorly towards the tips of the rete pegs to form committed transit amplifying cells (TACs). The TACs are highly proliferative (thus the designation “amplifying”) and are normally restricted to one to two basal cell layers. Upon completion of mitotic division, they progress to terminal differentiation in a highly orchestrated manner. The cells that are destined for differentiation undergo critical changes in their hemidesmosomal attachment (α6β4 to α3β1 integrins) that results in the cessation of cell division and almost immediate morphological changes as they transit upward into the spinous layer.
Stratum spinosum/spinous layer
Once the migrating basal keratinocytes transit into the immediately suprabasal region of the several cells-wide stratum spinosum they assume the appearance of spinous cells. Larger than basal cells and with a progressively lower nucleus-to-cytoplasm ratio, spinous cells are ovoid or stellate rather than cuboidal in shape. On conventionally stained sections (hematoxylin and eosin), they demonstrate abundant eosinophilic cytoplasm with a progressively shrinking nucleus as they approach the surface (Figures 9.8, 9.11a). In addition, they are characterized by distinctive spine-like projections (prickles, acanthe) on the surfaces of their respective plasma membranes. The spines represent extensions into the intercellular space of cell processes bearing desmosomes, the elaborate, tufted biochemical and mechanical attachment apparatus that links individual keratinocytes to adjacent keratinocytes. Desmosomes are intracytoplasmic placodes comprised of proteins (desmoplakins and plakoglobin) located on the inner aspect of the plasma membrane (Figure 9.12). Within the cytoplasm, a braid-like weave of intermediate keratin filaments attaches itself to the desmosome. From the intracytoplasmic placode desmoglein and desmocollin (paddle-like proteins from the cadherin family of attachment proteins) penetrate the intercellular space. They form Velcro-like linkages with their counterparts on the surface of neighboring cells to create robust intercellular bonds. This attachment apparatus creates the intercellular bridges visible on light microscopy (Figure 9.11).
Spinous cells are, by definition, post-mitotic and nonproliferative at this point. The changes in morphology described above are accompanied by and a direct result of (1) the accumulation of a constantly changing range of keratin filaments, (2) change in the nature of basal domain attachment, (3) activation of apoptotic mechanisms, (4) loss of cell organelles, (5) chromatin condensation/breakdown (pyknosis/karyorrhexis), and (6) protein crosslinking across cell membranes (keratin and involucrin complexes). This spectrum of change ultimately eventuates in exfoliation as keratotic debris (Figure 9.7) within the stratum superficiale.
Stratum superficiale/stratum corneum/cornified layer/keratin layer
The stratum superficiale (cornified layer/keratin layer) is the defining apical domain and the maturational end-point of stratified squamous epithelium. It is characterized morphologically by the presence of flattened epithelial cells. This layer can vary both in width and degree of surface keratinization (nonkeratinized, parakeratinized, or orthokeratinized), depending upon anatomic location, the degree of stimulation from friction, or both. Cells may be anuclear or contain highly condensed “pyknotic” nuclei (Figure 9.13). At this point the cells have lost most cytoplasmic organelles and have accumulated predominantly cytokeratins, forming a dense protein-rich meshwork with tight intercellular junctions. Prior to the cells being shed into the oral cavity, it is these tight intercellular junctions that provide critical water resistance and protection. These cells also provide a surface for coating with protective salivary proteins.
Stratum superficiale – nonkeratinized, orthokeratinized, parakeratinized
By light microscopy the stratum superficiale in the oral cavity can be classified as being nonkeratinized, orthokeratinized, or parakeratinized (Figure 9.13). Irrespective of the degree of keratinization, there is considerable variation in the width of the stratum superficiale among various oral mucosal locations. Oral mucosal surfaces like the attached gingiva and the hard palatal mucosa as well as the dorsal tongue are covered by a dense layer of surface keratin. These locations are subjected to considerable frictional force and stimulation during the process of mastication, and are consequently referred to as “keratinized” oral mucosa. In contrast, the remaining oral mucosal surface epithelium, which comprises the lining mucosa, is referred to as “nonkeratinized” mucosa.
Nonkeratinized squamous epithelium lines the greatest surface area of the oral mucosa, and includes the buccal and labial mucosae, the alveolar and vestibular mucosae, soft palate, tonsillar pillars, floor of the mouth, and ventral and lateral surfaces of the tongue (Figure 9.13). The epithelium covering the fungiform, foliate, and circumvallate papillae (three of the four types of specialized dorsal tongue mucosae) is similarly described. The designation of these epithelia as “nonkeratinized” is misleading, given that all keratinocytes contain abundant intracytoplasmic keratin filaments. As oral squamous cells differentiate and mature in their migration from the stratum basale upward and through more superficial layers, the composition of the keratin filaments unique to the keratinocytes in each epithelial stratum changes. When compared to their “keratinized” counterparts, keratinocytes from “nonkeratinized” mucosal surfaces contain sparsely distributed and less fasciculated bundles of tonofilaments. On electron microscopy, the tonofibrils in keratinocytes from keratinized surfaces are tightly bound and dense. Additionally, the cells within the superficial layers tend to be much larger in nonkeratinized mucosae. The composition of the glycolipid rich membrane-coating granules of keratinized versus nonkeratinized epithelium is different. As the membrane-coating granules fuse with the cell membranes of both keratinized and nonkeratinized mucosal epithelium, they release their contents into the intercellular spaces to produce a dynamic membrane. This controls the permeability of water soluble substances through the superficial keratin (cornified) layer. In keratinized mucosa, this is a more effective barrier against water loss and ingress of various chemicals, including noxious substances. Therefore, while tissue sections from keratinized and nonkeratinized tissues demonstrate morphological differences, their respective designations are misleading. In reality nonkeratinized epithelium actually is “keratinized,” but to a significantly lesser degree than keratinized epithelium.
Orthokeratin is a term used to designate keratin that stains deeply with eosin and consists of flat dehydrated cells that lack nuclei or retain ghost-like nuclear remnants (Figure 9.13). It often has a basket-woven appearance and is the keratin that is seen on epidermal surfaces. Within the oral cavity, orthokeratinized epithelium is seen primarily on attached mucosal surfaces. In addition to the surface characteristics, it has a conspicuous granular cell layer, the stratum granulosum, just below the stratum superficiale. Stratum granulosum cells appear flatter than spinous cells, with ovoid nuclei and conspicuous basophilic keratohyalin granules that occupy their cytoplasms (Figure 9.13). The appearance of these globular granules, composed of filaggrin and trichohyalin, signals the initiation of the final stage of squamous apoptosis. It results from a rapid drop in pH (7.17 to 5.0), with consequent desmosomal degradation and complete breakdown of nuclear structures and organelles (Figure 9.7).
Parakeratin describes a keratotic surface in which nuclei are retained and appear shrunken, spindly, and dark (pyknotic) (Figures 9.13, 9.25). On H & E staining, parakeratinized epithelium is paler and relatively more eosinophilic than orthokeratin. Additionally, in parakeratinized epithelium there is no stratum granulosum evident on light microscopy (Figures 9.13, 9.25). Parakeratinized epithelium is seen surfacing the filiform papillae, vermilion border of the lip, dorsal tongue, and in areas where there is relatively rapid turnover of epithelium.
The epithelial-stromal interface
The basal-most layer of epithelial cells is attached to a basement membrane, an interface zone that separates the epithelium from the papillary layer of the lamina propria, where the capillary-rich, loose, thin fibrocollagenous tissue is arranged in papillary (finger-like) upward projections that interdigitate with the epithelial rete pegs (Figure 9.8). Because squamous epithelium itself is avascular, this intimate hand-in-glove relationship between the epithelium and the connective tissue immediately beneath it provides for the greatest possible surface area for nutrient exchange and waste elimination, intercommunication, dispersion of mechanical forces that are exacted on the epithelial surface, immunologic protection, and sensation. The length, width, and number of rete pegs vary with respect to oral location and the functional demands exacted on the mucosa in that site. For example, there are more rete pegs per unit area in attached gingival and hard palatal mucosa (masticatory mucosa) than in the buccal mucosa (lining mucosa).
On light microscopy the basement membrane appears as an uncomplicated line of demarcation between the overlying epithelium and the underlying lamina propria. This apparent simplicity is deceptive: electron microscopy reveals an elegantly organized and complex construction, the basal lamina. The basal lamina consists of two layers, an electron-lucent lamina lucida adjacent to the basal membrane of the epithelial cells, and the lamina densa. The lamina densa is a distinct, coarse, electron-dense web of filaments comprised of a meshwork of extracellular matrix proteins that forms an anchoring substrate for the hemidesmosomes of the overlying cells. This foundation consists primarily of laminins (laminin 5), type IV collagen, perlecan, and several other glycoproteins and proteoglycans (Figure 9.12).
The hemidesmosomes in stratified squamous epithelium are composed of a heterodimer, α6β4 integrin that links the intermediate filament cytoskeleton to the basement membrane meshwork. As described above, linking proteins like BP230 and BP180 (collagen X/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


