Chapter 10
Chemoreception and Perception
Marion E. Frank
Department of Oral Health & Diagnostic Sciences, School of Dental Medicine, University of Connecticut
Oral chemoreception includes the senses of taste (gustation), smell (olfaction), and the chemical somatic senses, which will be covered in Chapter 9, ”Oral Mucosa and Mucosal Sensation,” and Chapter 12, “Orofacial Pain, Touch and Thermosensation, and Sensorimotor Functions.” This current chapter, not solely a compilation of current knowledge on the gustatory and olfactory systems, integrates into the text experimental techniques used to develop that knowledge. Perceptual (human objective and subjective psychophysics, animal conditioned taste aversion and preference), physiological (receptor potential, multi- and single- neuron electrophysiology; two-photon calcium imaging in vivo, single dissociated olfactory sensory neuron (OSN) calcium imaging, event-related fMRI brain imaging) and morphological (light and electron microscopy) methodologies are cited.
The independent senses of taste and smell have evolved to monitor chemical environments for health and wellbeing. With them, as well as general somatosensations, distinct sources of chemosensory information, it can be decided that foods need more or less sugar or salt, whether the scent of dinner is foul or appetizing, and whether lemonade is too sharp. One of the first things learned from patients at the opening of the Connecticut Taste and Smell Clinic in 1980 was that people often confuse smells with tastes. They may feel certain that they cannot taste after an automobile accident involving head trauma but have no measurable loss of gustation. They are perfectly capable of distinguishing salt from sugar. Retronasal smelling, a recent topic of interest, helps explain the patients’ confusions. Odors from the outside are sensed orthonasally during inspiration; but on expiration oral odors are sensed, retronasally. (See also Disorders in Glossary). Retronasal odors from the mouth, closely allied with tastes, contribute to the specific flavor of a food such as asparagus, which also gives urine an unusual orthonasal odor.
Tastes and smells of chemosensory compounds are attached to innate and developed preferences and aversions. Odors’ primal pleasure/displeasure dimension (for example) is hardwired in olfactory mucosal patches of sensitivity to volatile compounds with appealing (+) or repulsive (–) odors. This is elaborated on in Box 10.1(1). Also, single chemosensory compounds may activate several chemosensory systems. For example, acetic acid has a vinegary smell, a sour taste, and painfully irritates as concentration increases. Anosmic individuals, without a sense of smell, can detect acetic acid orthonasally but only at a higher concentration than normosmic individuals. Correspondingly, individuals taste-blind for specific compounds such as phenylthiocarbamide (PTC) and related bitter compounds detect them only at high concentrations, as shown in Box 10.2(1). Moreover, chemosensory stimulus recognition is fleeting, fading with time through peripheral adaptation and mutual central inhibition evident in mixture suppression among separate neural pathways. This coding strategy emphasizes the identification of recently appearing and strong tasting or smelling chemical compounds.
In this chapter, the structure and function of the two chemosenses of taste and smell are covered in separate sections. A final section addresses clinical issues.
Taste
Fundamentals and historical perspective
Taste evaluates beneficial and dangerous water-soluble chemicals taken into the mouth to assist nutriture through the pleasure of eating and poisoning through the awfulness of tainted foods.
A century ago, Hans Henning represented the relationships between pure human taste perceptions geometrically on the surface of a tetrahedron: “primaries” at the four corners and mixtures of two or three taste “primaries” elsewhere on edges and faces (Fig. 10.1). In the tetrahedron drawn, compounds with each taste (sweet, salty, sour, and bitter) are positioned at corners, compounds with binary mixtures of tastes (saccharin, KCl, NH4Cl) are positioned appropriately on edges between pure tastes, and a compound with a mixture of three tastes (NaSO4) is positioned on a tetrahedral face. Single chemical compounds with one taste quality, single compounds with two or three taste qualities, and, by extension, mixtures of two or three compounds with different pure qualities are represented by the tetrahedral model.
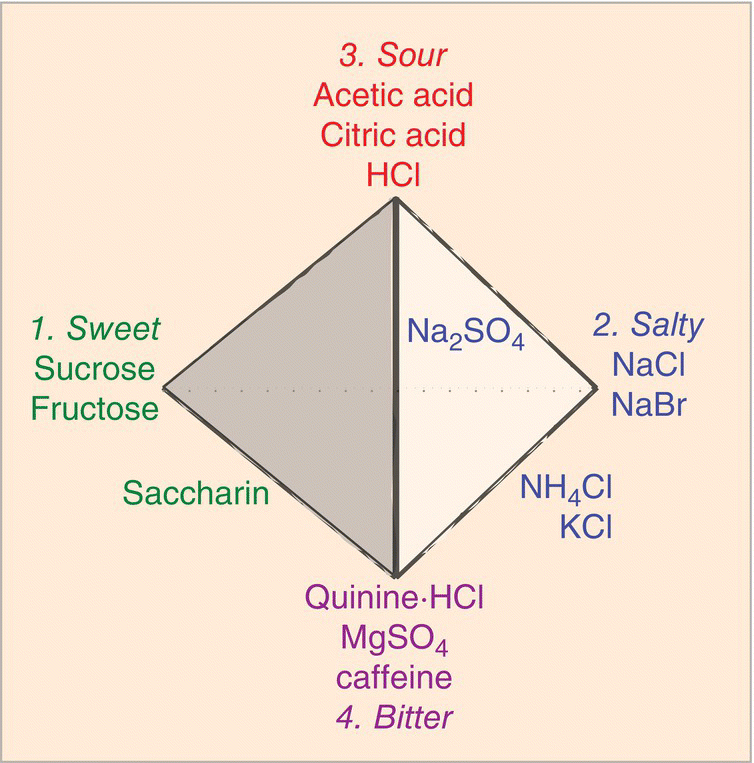
Figure 10.1 Henning’s 1916 taste tetrahedron: compounds with pure taste primaries at corners (1, 2, 3, 4), mixtures on edges or faces (not in the interior because it was thought no stimulus was a mix of the four tastes). (See also quality coding in Glossary). Taste stimuli were dispersed on the surface of the tetrahedron according to how similar they tasted to primaries.
Chemical stimuli and taste quality
Sugars, some amino acids, and artificial sweeteners such as saccharin and aspartame are sweet. Halide sodium salts NaCl and NaBr are salty. Non-sodium halide salts are salty and bitter. KCl, NH4Cl, MgSO4 and salts with large organic cations such as denatonium benzoate and quinine hydrochloride and zwitter-ionic amino acids as well as nonionic alkaloids (thioureas, bile acids and glycosides) are bitter, a broad category of unpleasant tastes that may include several distinct experiences. Sour-tasting acids, which are also irritants, may also have characteristic odors, such as the vinegary acetic acid. Ethanol is the epitome multi-chemosensory stimulus, tasting bitter and sweet and having an odor, as well as being an irritant. The non-gustatory aspects of taste stimuli were intentionally not considered in Henning’s pure-taste tetrahedron. Structures of a variety of water-soluble compounds with a broad range of tastes are shown in Fig. 10.2.
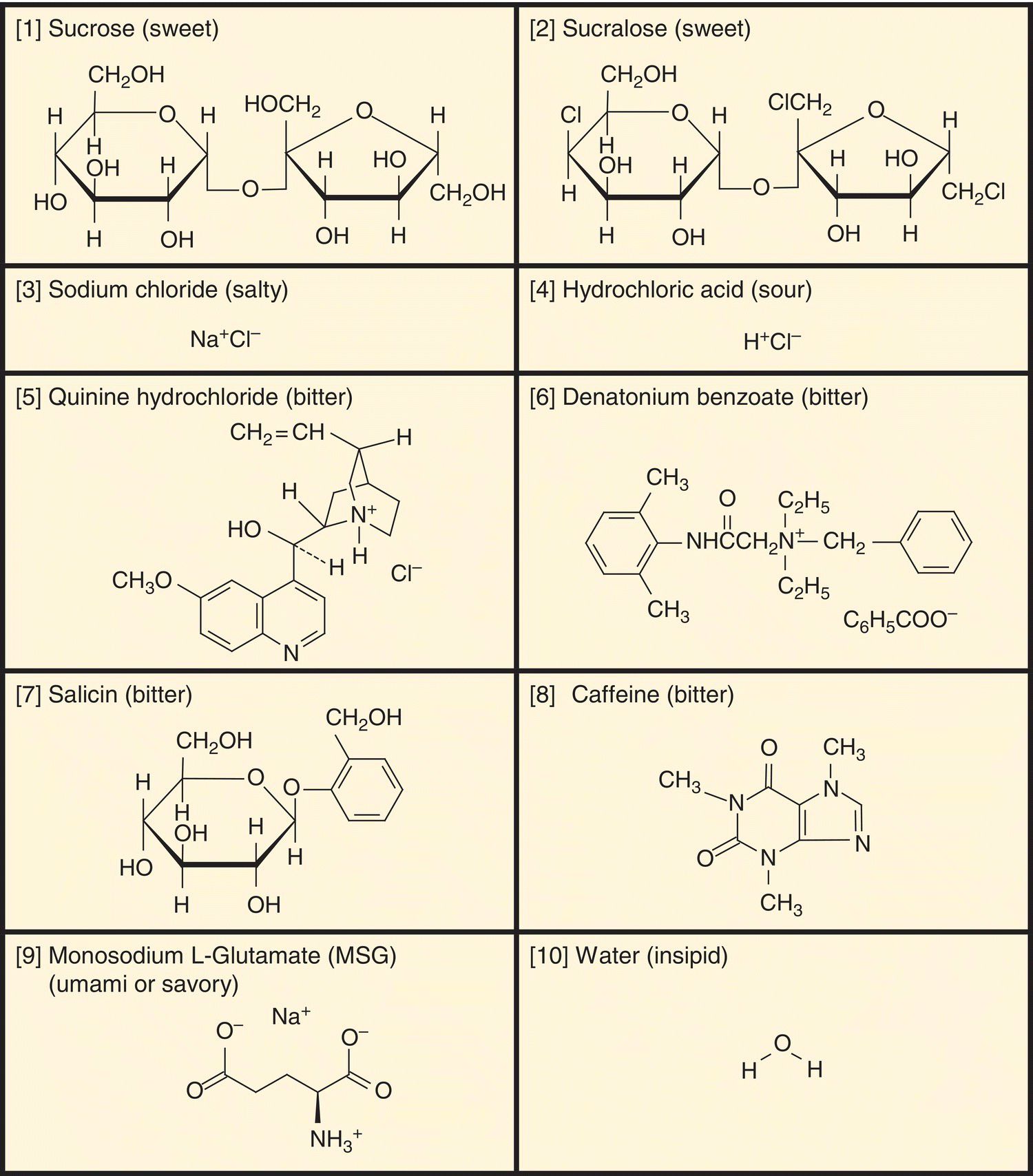
Figure 10.2 Chemicals representative of basic taste qualities. (1) Sweet sucrose occurs in sugar cane, maple sap, and many flowers and fruits; (2) sweet sucralose is an artificial sweetener derived by replacing three sucrose OH groups by Cl atoms. (3) Salty sodium chloride is the only chemical that is pure salty. (4) Sour hydrochloric acid is one of many acids producing sour H+ ions. (5) Bitter quinine hydrochloride is a natural alkaloid from cinchona tree bark; (6) bitter denatonium benzoate is an extremely bitter synthetic chemical; (7) bitter salicin is a natural glycoside from willow tree bark and ligand of the T2R16 receptor; (8) caffeine is a weakly bitter natural alkaloid from coffee and tea. (9) Umami (savory) monosodium glutamate is a natural amino acid that is also produced synthetically. (10) Insipid (tasteless) is the taste of water, likely due to contrast with saliva. (See also Taste Stimuli in Glossary).
(From Hettinger, T.P. (2013) Unpublished.)
Tastes surely have four pure qualities that signal nutritious carbohydrates and minerals or harmful substances. Another taste, savory (umami in Japanese), has been legitimized with discovery of a receptor. It is evoked by monosodium L-glutamate (MSG), or better, a mixture of MSG and inosine monophosphate (IMP), and may signal protein sources. Other stimulus domains, free fatty acids (for example, linoleic acid), glucose polymers (for example, the nutritional supplement Polycose®), and low osmolarity (water) may also be detected orally; possibly with involvement of gustatory receptors.
Species-specific taste worlds
Species differences in the gustatory quality of chemicals can be great, especially for the many compounds with bitter tastes associated with the T2R bitter genes. The variation may reflect the importance of detecting the different poisons and food items used for nourishment as habitats change and species evolve. For example, carnivorous felines are unable to taste sugar, which is consistent with pseudogenization of the T1R2 sweet-taste receptor gene during evolutionary time owing to a release from positive evolution. Conversely, ethanol (bitter-sweet and poisonous to humans at high concentrations) is primarily sweet to golden hamsters, who utilize its caloric value without inebriation poisoning when feeding on fermented grain stored in their deep burrow domiciles.
Studies on rodents are used as models for human taste. However, before linking human to animal taste, differences in perceptual “taste worlds” must be experimentally addressed. To this end, confusability of taste stimuli, quantitatively measured psychophysically in humans with taste confusion matrix methodology and behaviorally in animals with taste aversion generalization tests, reveals how taste mixtures are evaluated. Multivariate analysis of human confusions, Box 10.3(1), distributed the prototypic sweet sucrose, salty NaCl, sour citric acid, bitter quinine, and five binary taste mixtures in a two-dimensional space. All taste stimuli were distinct from insipid water, some mixtures fell between mixture components but some were much closer to a dominant component. Correspondingly, intake suppression seen in hamster generalization tests, Box 10.3(2), differed for hamsters that learned aversions to three individual compounds (sucrose, NaCl, quinine) and three binary or one ternary mixture of the compounds. Like humans, the hamsters detected components in binary mixtures, and the ability to recognize components deteriorated with the higher level ternary mixture, mixture suppression reminiscent of human tasting. (See also Fig. 10.10 under “Peripheral Nerves” below.)
Multivariate analysis of hamster taste generalizations resulted in the “taste world” shown in Fig. 10.3, which is to be compared to the human taste tetrahedron in Fig. 10.1. The quantitative multivariate analysis shows hamsters, as humans, perceive four qualities. In the hamsters’ world, sweeteners and sour acids fall in distinct regions; however, the two other hamster tastes, one labeled ‘Na salts’ and the other ‘bitter salts’, differ from human tastes, demonstrating critical species variation regarding tastes of salts and bitter compounds. These differences between rodents and humans have impeded understanding of human salty and bitter tastes. Nonetheless, the multivariate taste-world model shows equivalence to Henning’s tetrahedral model with four taste groupings in three-dimensional space. The objectively determined multidimensional scaling (MDS) configurations are more general, with the mathematical precision to exactly position components and mixtures in one multidimensional space.
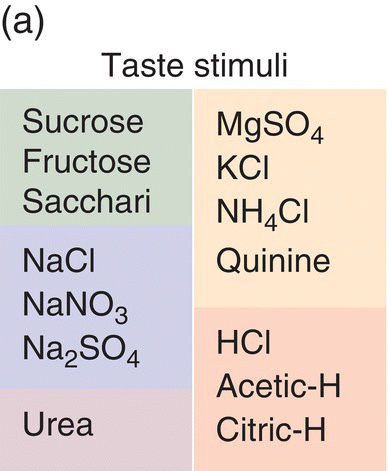
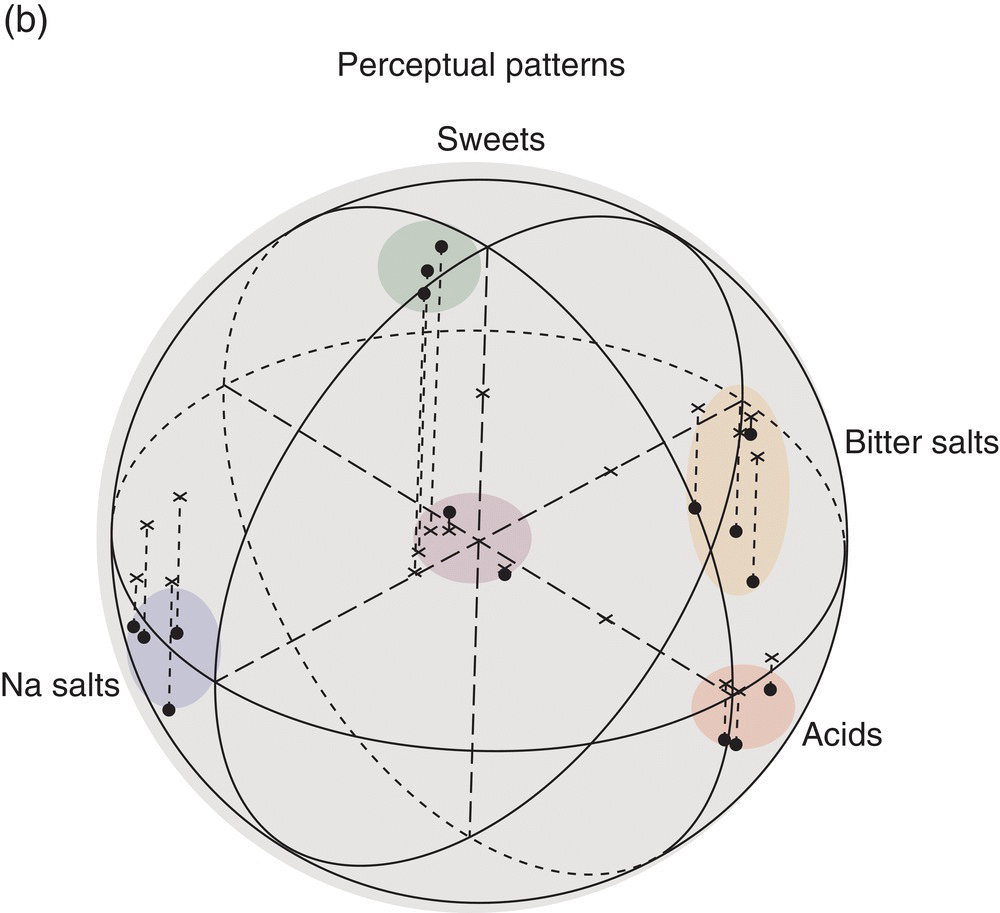
Figure 10.3 Behavioral discriminations of 14 taste compounds (a) used to identify qualitatively distinct perceptions in the hamster taste world (b). The spherical 3D multidimensional scaling (MDS) of generalizations of conditioned taste aversions (CTA), quantitatively based on percent drinking suppression values, identifies stimuli that hamsters confuse with one another, which are represented by points that are near one another. (See also Taste Dimensions in Glossary). Two of the points falling among Na salts represent two Na2SO4 concentrations. Two points at zero, the center of the sphere, represent two urea concentrations that were unaccounted for by this solution.
(From Frank, M.E. & Nowlis G.H. (1989) Learned aversions and taste qualities in hamsters. Chemical Senses, 14, 379–394. Reproduced with permission from Oxford University Press.)
Studies of stimulus identification provide insight into real-world chemosensory processing by drawing attention to the human capacity for dynamic stimulus analysis of the shifting chemicals in complex mixtures. Table 10.1 presents an experimental protocol designed to study human dynamic chemosensory analysis in which either taste or smell stimuli are presented one after the other in pairs. The first stimulus, sampled for 5 seconds, is followed by the second stimulus to be identified. Independent stimuli, easily identified following water, are less accurately identified in a mixture due to mixture suppression, as specified in the “not-adapted” case 1 in Table 10.1 and in Fig. 10.4, which presents results from an experiment with sucrose and sodium chloride (NaCl). Their sugar or salt tastes do not cross-adapt; that is, do not affect one another’s identification when sequentially applied. Cases 2, 3, and 4 in Table 10.1 deal with selectively adapting individual component stimuli, which influence mixture-component identification as shown for tastes in Fig. 10.4. In essence, un-adapted extra components regain quality identity suppressed in mixtures (case 2), while adapted ambient component identities are further suppressed (case 3). When a number of stimuli are non-selectively pre-adapted together (case 4), no stimulus dominates, but all merely self-adapt as they do as single compounds. Apparently, mixture suppression, rather than obscuring quality information, is likely a central perceptual mechanism that adjusts effective concentrations. This process, combined with rapid sensory adaptation, makes it possible to identify single stimulus compounds detected by individual chemosensory receptors. The experimental findings, consistent with everyday experience, highlight the importance of identifiable characteristic of tastes and odors in the processing of chemosensory compounds.
Table 10.1 Prior selective adaptation of ambient compounds aids identification of tastes or odors of extra mixture components in humans.
| Stimulus Pairs Presented | Tastes or Odors Identified | |||
| Single Compound (A1) | Mixture Component (A2) | Percentage Correct (A1 or A2) | ||
| 1 | Not-Adapted | |||
| 0→A1 | 0→A2B | A2 < A1 | Mixture Component < Single Compound | |
| 2 | Extra (Selectively Adapted) | |||
| 0→A1 | B→A2B | A2 = A1 | Extra Mixture Component = Single Compound | |
| 3 | Ambient (Selectively Adapted) | |||
| A→A1 | A→A2B | A2 < A1 | Ambient Mixture Component < Self-Adapted Single Compound | |
| 4 | Mixture (Non-Selectively Adapted) | |||
| A→A1 | AB→A2B | A2 = A1 | Mixture-Adapted Mixture Component = Self-Adapted Single Compound | |
Identifications of tastes or odors of binary-mixture components (A2) are compared to identifications of single-compounds (A1). A and B are chemical stimuli that elicit A and B independent tastes or odors; 0 = water. Identification cases 1 & 2: Single compound after water vs. mixture component after either (1) water or (2) another mixture component. Identification cases 3 & 4: Single compound after the same compound vs. mixture after (3) one component or (4) both components. (See also Mixture Suppression in Glossary). Data for each of the four cases are shown for a taste experiment in Fig. 10.4. (See Adaptation in Glossary). (Derived from data in Frank, M.E., Goyert, H.F., Formaker, B.K., & Hettinger, T.P. (2012) Effects of selective adaptation on coding sugar and salt tastes in mixtures. Chemical Senses, 37, 701–709; and Frank, M.E., Goyert, H.F., & Hettinger, T.P. (2010) Time and intensity factors in identification of components of odor mixtures. Chemical Senses, 35, 777–787.)
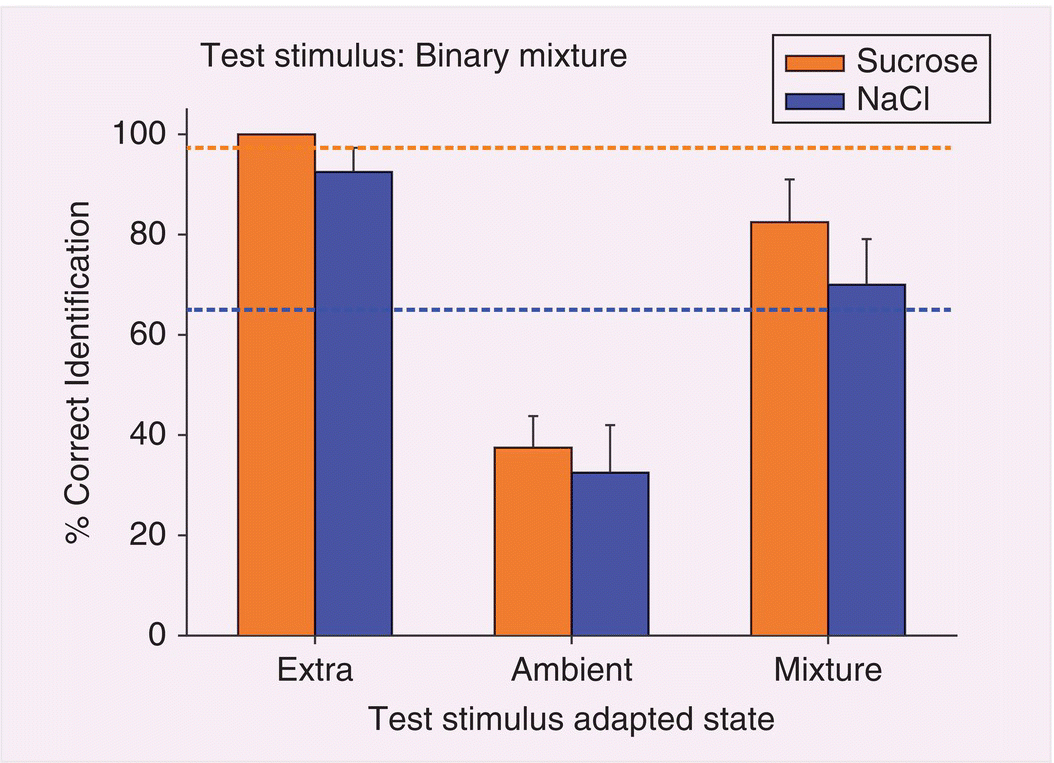
Figure 10.4 Taste mixture component identification after selective and nonselective adaptation in humans. Sucrose and NaCl have independent tastes that are perfectly (100%) identified as sugar and salt following a water rinse. However, identification of the tastes as mixture components fluctuates, as delineated in Table 10.1. Tastes of extra mixture components (case 2 in Table 10.1) are also identified nearly perfectly. However, tastes of selectively adapted ambient mixture components (case 3 in Table 10.1) are poorly identified, more so than when the mixture is preceded by the mixture (case 4 in Table 10.1). (See also Mixture Suppression in Glossary). Dotted lines reveal levels of identification of component tastes in the mixture preceded by water (case 1 in Table 10.1).
(From Frank, M.E., Goyert, H.F., Formaker, B.K., & Hettinger, T.P. (2012) Effects of selective adaptation on coding sugar and salt tastes in mixtures. Chemical Senses, 37, 701–709. Reproduced with permission from Oxford University Press.)
Peripheral activation
Taste buds detect water-soluble chemicals
About 50 taste cells (TC) reside in taste bud cellular clusters located in lingual epithelia and elsewhere in oropharyngeal mucosa. Taste cells turn over every 10 days, which reflects their epithelial origin. Fig. 10.5a is a photomicrograph of a taste bud within a fungiform papilla. Mature taste buds depend on neurotrophism; atrophied anlage remain after injury to the taste nerve. Taste-bud cells project membrane extensions, microvilli, into the gustatory pore, which contains dense mucosubstance, as pictured in the diagram in Fig. 10.5b. Type I, II, and III TC with different lineages each have unique morphologies and biochemical features, suggestive of distinct functions. Glial-like, GLAST (glutamate aspartate transporter) containing, type I dark cells with sheet-like processes intermingle among other TC, possibly serving physical and biochemical supporting roles. Type II TC (light cells) contain the biochemical necessities for taste stimulus transduction. This includes the G protein α-gustducin, T1R and T2R G protein-coupled receptors (GPCR), as well as downstream elements phospholipase C (PLCβ2) and transient receptor potential (TRP) cation channel TRPM5 necessary for TC depolarization. All must be operational for the tasting of sweet, savory and bitter compounds, which is elaborated on in the next few paragraphs. Type III TC, which project one broad apical microvillus into the gustatory pore and may be directly activated by ionic taste stimuli, are the only TC known to be synaptically associated with the axons of taste nerves at the base of the taste bud. Multiple trigeminal nerve (CN V) endings are seen apically in the papilla outside the taste bud that are capable of mediating touch, temperature and chemical irritation via TRP channels, a topic covered in Chapter 9, “Oral Mucosa and Mucosal Sensation.”
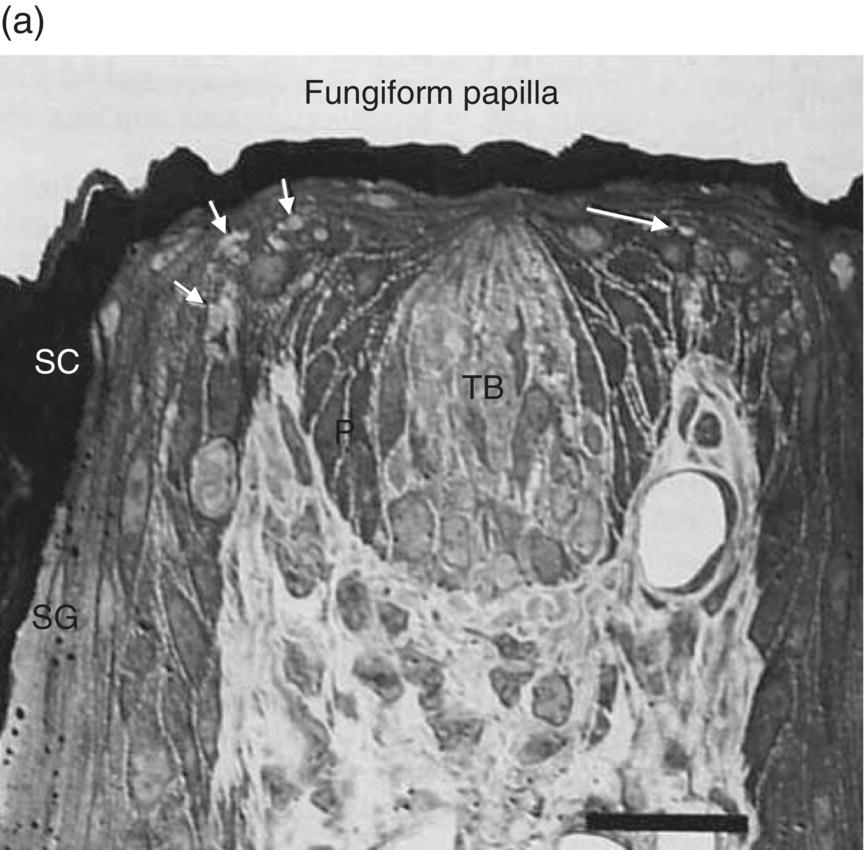
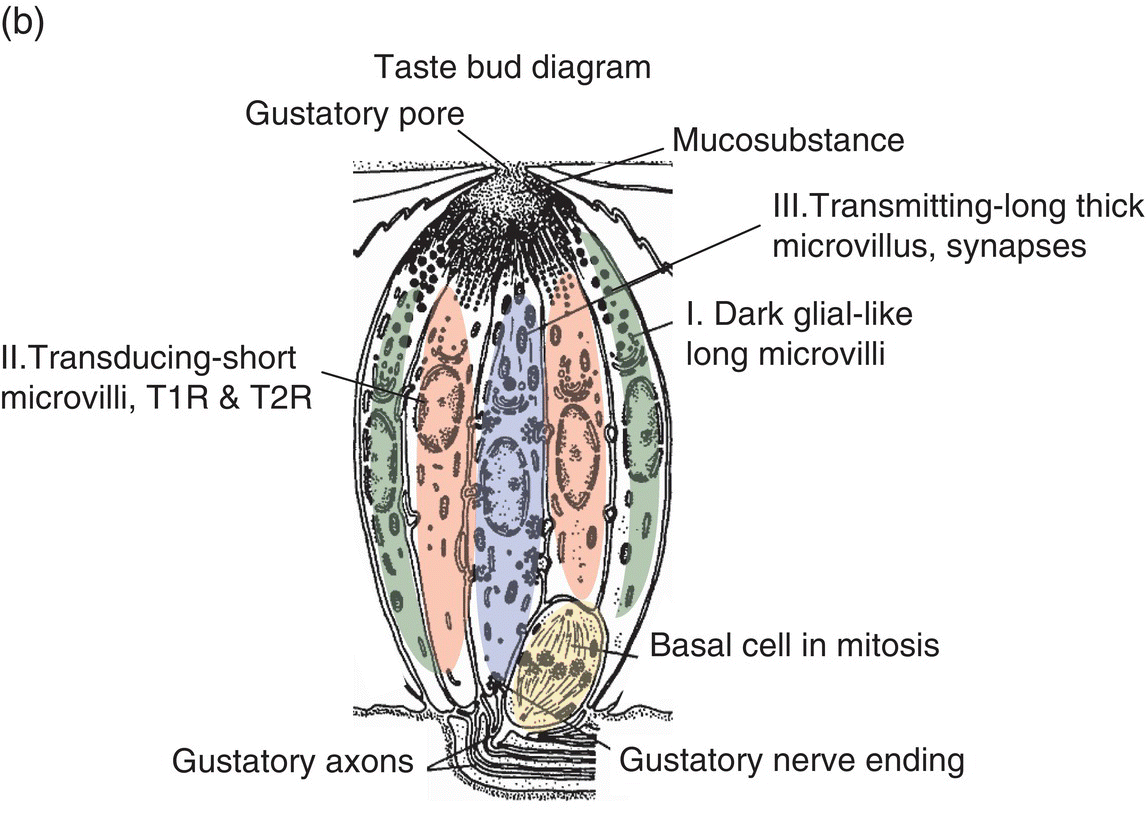
Figure 10.5 (a) Fungiform papilla and (b) taste bud diagram. In photomicrograph (a) of a toluidine- blue stained, 1-μ-thick longitudinal section through a taste bud, TB = taste bud, SC = stratum corneum, SG = stratum granulosum. Arrows point to trigeminal lingual nerve endings outside the taste bud. In diagram (b), the gustatory pore is drawn at the apical end of the taste bud where stimuli interact with receptors in microvillar membranes. Three morphological types of taste cells (I, II, III) are depicted in the body of the taste bud. At the base of the taste bud, a basal cell and gustatory nerve endings are drawn.
(a): From Whitehead, M.C., Beeman, C.S., & Kinsella, B.A. (1985) Distribution of taste and general sensory nerve endings in fungiform papillae of the hamster. American Journal of Anatomy, 173, 185–201. (b) From Banister, L.H. (1976) Sensory terminals of peripheral nerves. In: The Peripheral Nerve (ed. D.N. Landon), pp. 396–463. Chapman & Hall, London. Reproduced with permission from John Wiley & Sons.)
Taste receptors are related to perceptual taste quality
Interactions between taste stimuli and taste receptors (TR) occur within gustatory pores. Nonionic taste stimuli first bind to receptor proteins in apical membranes of TC microvilli that are coupled to second-messenger systems. Members of two families of GPCR detect taste stimuli, 3 T1R and 30 T2R. The G protein gustducin with an α subunit quite similar to retinal rod transducin (which can substitute for gustducin) is ubiquitously involved. Sweet ligands activate the single heterodimeric GPCR T1R2/T1R3; its structure is drawn in Fig. 10.6. Umami (savory) ligands may activate heterodimer T1R1/T1R3. Each of these receptors is restricted to a distinct subset of taste cells. Structurally varied bitter ligands activate the more numerous T2R that appear together in a single TC variant. Most human T2R genes are tightly clustered on chromosomes 12 or 7. T2R38 on chromosome 7q36 is one of few human bitter receptors for which function is definitively established due to discovery and documentation of variation in tasting PTC and related compounds nearly 80 years ago. Of the two common haplotypes, PAV, found in primates, is thought to be the ancestral. (See also Box 10.2(2) above.) Stimulus binding of ligands such as sweet sucralose and bitter salicin is associated with extracellular portions of T1R2 and T2R16, respectively.
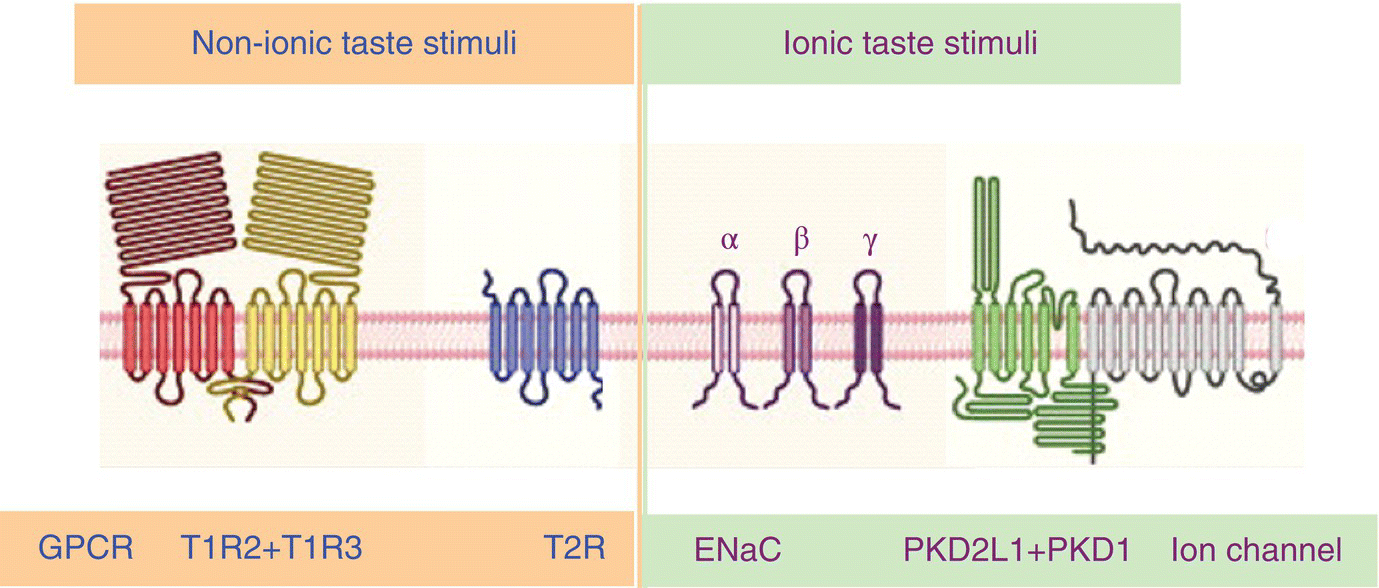
Figure 10.6 Taste bud cells are activated either indirectly by GPCR or directly through ion channels. Sweet sugar acts via heterodimeric GPCR T1R and bitter quinine acts via GPCR T2R; salty NaCl depends on ENaC ion channels, and sour citric acid depends on heterodimeric PKD TRP ion channels. Each eventually initiate neural activity in four independent peripheral taste pathways.
(From Yarmolinsky, D.A., Zuker, C.S., & Ryba, N.J. (2009) Common sense about taste: from mammals to insects. Cell, 139, 234–244. Reproduced with permission from Elsevier.)
Ionic taste stimuli directly activate dedicated subsets of TC by passing through ion channels in cell membranes, but the TC types involved are uncertain. In rodents and humans, a Na+ taste is transduced by influx of Na+ through an amiloride-sensitive ion channel (ENaC); its structure is drawn in Fig. 10.6. Box 10.4(2) below shows a hamster nerve recording demonstrating the effect of 10-μM amiloride on a response to 100-mM NaCl. The taste of NaCl is not completely accounted for by ENaC sensitivity; a quinine-like taste remains. Sour taste may be generated by influx of H+ through TRP PKD2L1 + PKD1 channels (structure drawn in Fig. 10.6) in membranes of type III TC. It also has been suggested that intracellular acidification has a role in the production of acid taste.
Transduction of nonionic and ionic taste stimuli alike concludes with TC depolarization, which results in activation of gustatory sensory neurons (GSN). Taste nerve responses and behavioral detection of stimuli of all taste qualities depend on adenosine 5’-triphosphate (ATP). Also, within the taste bud, before transmission to the brain, taste stimuli evoke release of serotonin, γ-amino butyric acid (GABA), and norepinephrine, chemicals capable of inhibiting or enhancing independently coded TC messages. The inhibition may provide safety control by reducing effective concentrations of contaminated nutrients.
Taste buds occur in several oral receptive fields
Lingual taste buds reside in multiple fungiform papillae, an example shown in Fig. 10.5A, distributed over the dorsal anterior two-thirds of the tongue, in foliate papillae on the lateral edges and circumvallate papillae on the dorsal surface of the posterior one-third of the tongue, as well as on the anterior soft palate and oropharynx, especially the upper laryngeal side of the epiglottis. Locations of the taste bud fields are diagrammed in Fig. 10.7. Frequency distributions of taste buds in mucosa of the lingual and palatal oral cavity and oral pharynx, from which three cranial nerves (CN), VII, IX, and X, in three peripheral ganglia (geniculate, petrosal, nodose) carry taste information to the brain are provided in Table 10.2. Taste buds are more common in foliate papillae and on the palate in hamster (and rat) than in humans. The species difference may relate to the masticatory function of the raised rodent intermolar lingual eminence during feeding. Epiglottal mucosal taste buds, at high proportion in human infants, are much rarer in 80 year olds even though peripheral gustatory function is generally well maintained with aging in humans. This leaves the upper airway less protected from aspiration of food/liquid at advanced age, which may lead to more frequent “choking.”
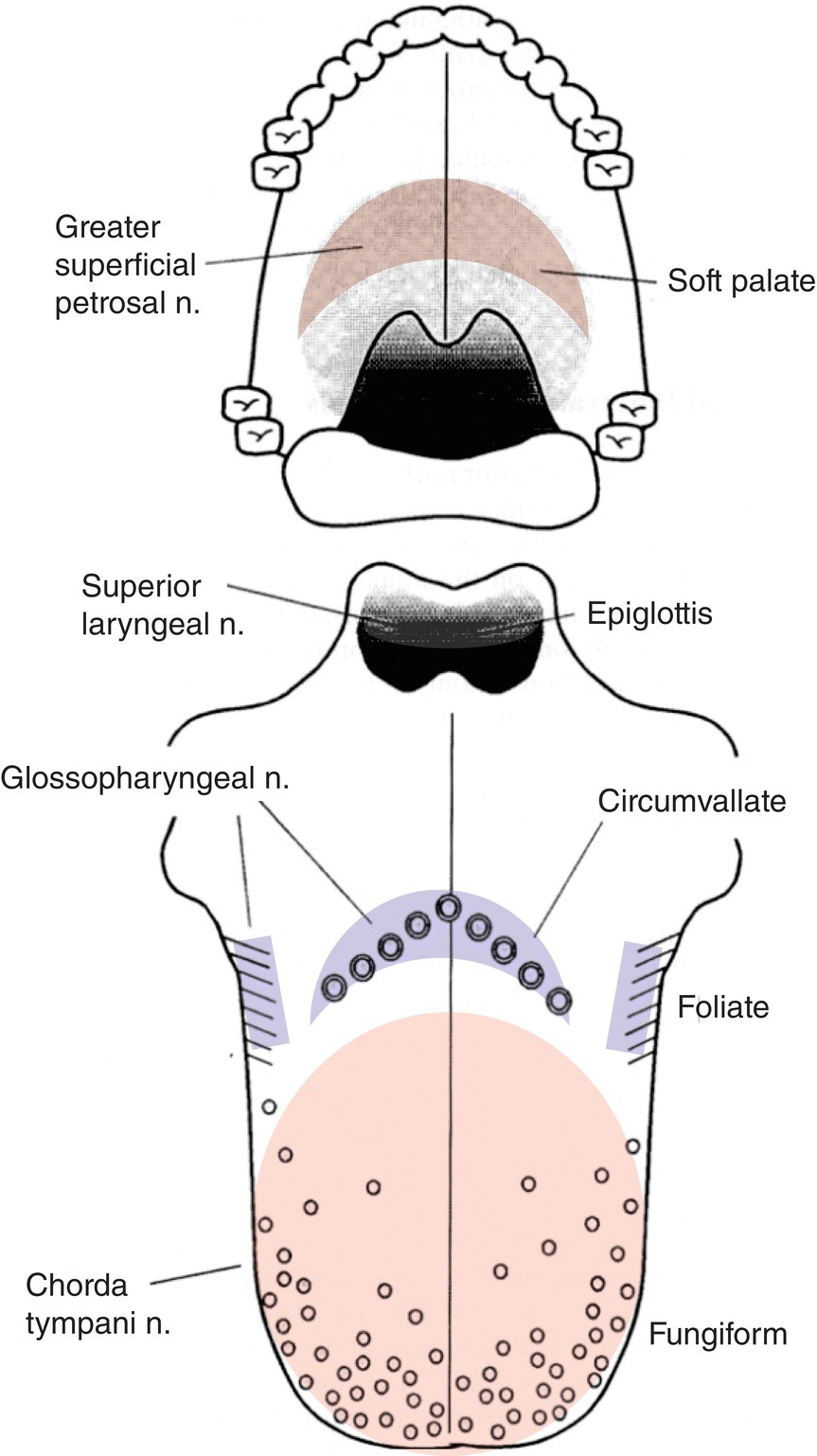
Figure 10.7 Diagram showing human taste bud fields. Fields innervated by CN VII are mostly at the tongue tip but also found on the soft palate. Fields innervated by CN IX are focused at the junction of oral and pharyngeal spaces. CN X innervates taste buds on the laryngeal face of the epiglottis. (See also Topographic in the Glossary).
(From St John, S.J. & Boughter, J.D., Jr. (2008) The gustatory system. In: Neuroscience in Medicine (ed. P.M. Conn), p. 604, Humana Press (Springer), New York. Originally published in Smith, D.V. & Shipley M.T. (1995) The gustatory system. In: Neuroscience in Medicine (ed. P.M. Conn) p. 513, JB Lippincott, Philadelphia. Reproduced with kind permission from Springer Science + Business Media.)
Table 10.2 The peripheral gustatory pathway
| % Total TB | |||||
| Region | Papillae | Human | Hamster | CN Branch | Ganglion |
| Tongue | VII. Facial | Geniculate | |||
| anterior 2/3 | Fungiform | 20% | 18% | Chorda Tympani | |
| Palate | Islands | 6% | 14% | Greater Superficial Petrosal | |
| Tongue | IX. Glossopharyngeal | ||||
| posterior 1/3 | Lingual | Petrosal | |||
| lateral | Foliate | 16% | 32% | ||
| dorsal | Vallate | 31% | 23% | ||
| Oropharynx | X. Vagus | Nodose | |||
| Epiglottis | Patches | *27% | 11% | Superior Laryngeal | |
Taste Buds (TB): Region, Cranial Nerve (CN) Branch, and Ganglion (G)
Total taste buds in human = 7902 (*in newborn); hamster = 723 (2% buccal wall/sublingual). (See also Chorda Tympani in the Glossary).
(Quantitative data from: Miller, I.J. & Smith, D.V. (1984) Quantitative taste bud distribution in the hamster. Physiology & Behavior, 32, 275–285; and Travers, S.P. & Nicklas, K. (1990) Taste bud distribution in the rat pharynx and larynx. Anatomical Record, 227, 373–379.)
In general, location and chemical sensitivities of each of the three CN taste-bud fields suggest distinct functions. CN VII, confined to the anterior portion of the tongue and palate, specializes in food selection by taste stimulus identification. CN IX, restricted to the posterior third of the tongue, specializes in reflexes of ingestion or rejection, whereas CN X taste buds specialize in airway protection. Studies of rodent behavioral discrimination after cutting individual taste nerves or applying specific inhibitors empirically verify this functional specialization. Thus, stimulus-associated reflex actions rather than taste-quality perceptions may be mapped somatotopically in the oral cavity.
Peripheral nerves
GSN are pseudo-unipolar neurons with cell bodies in the three peripheral ganglia listed in Table 10.2. Central and peripheral coursing axonal processes of the neurons are small, either myelinated or unmyelinated. GSN of CN VII travel in the chorda tympani (CT), which joins the lingual branch of the mandibular division of the trigeminal nerve (CN V) before reaching the tongue, and the greater superficial petrosal (GSP) nerve, which reaches the soft palate via the vidian nerve as traced in Fig. 10.8. Geniculate central axonal processes travel through the internal auditory meatus to enter the brainstem as the intermediate nerve root, which lies between CN VII and VIII roots. Petrosal central axonal processes of lingual sensory branches of CN IX and nodose central axonal processes of superior laryngeal branches of CN X enter the brain stem within the roots of CN IX and X, respectively.
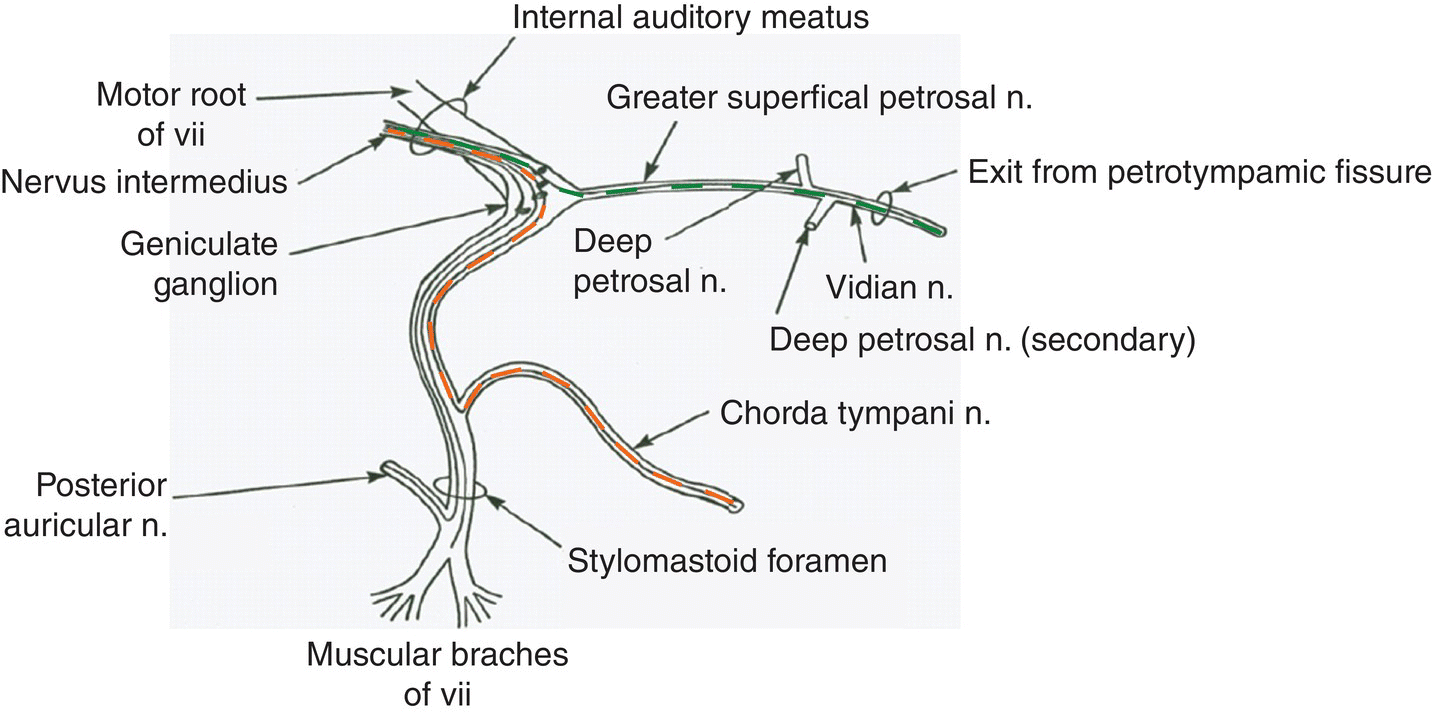
Figure 10.8 Primary afferent taste neurons in the sensory division of CN VII, the facial nerve. The peripheral and central coursing axons of the two sensory branches of CN VII from the rat geniculate ganglion are shown: the chorda tympani to the front of the tongue in orange, and the greater superficial petrosal to the soft palate in green. Centrally, they join to form the intermediate nerve root that enters the brain stem between CN VII and CN VIII
(From Gomez, M.M. (1978) The peripheral distribution of the rat geniculate ganglion. PhD Thesis, Wake Forest University, Bowman Gray School of Medicine, Winston-Salem NC. UMI: AAT 7906509.).
Visualization of the CT medial to the mandible coursing from the tympanic bulla to join the lingual nerve is achieved with removal of soft tissue after disarticulation of the temporomandibular joint (anatomy described in detail in Chapter 8, “Temporomandibular Joint”), with removal of its condylar and coronoid processes and the zygomatic arch. This surgical approach and, as important, the ease with which the fungiform taste buds on the tongue tip can be stimulated, has resulted in taste function being defined primarily for a single taste bud field. Box 10.4 contains more information on the techniques used for GSN electrophysiological analysis. Function of individual GSN is measured with single-unit recording (an example is included in Box 10.4(1)); whereas, gustatory sensory nerve function, the combined responses of hundreds of single GSN, is measured by whole-nerve recording (an example is included in Box 10.4(2)).
Gustatory sensory neuron function
Single hamster GSN respond selectively to taste compounds as illustrated for the CT in Fig. 10.9. The neural responses of single neurons in this one taste nerve are compatible with (but less complex than) perceptual “taste worlds” based on all oral sensory nerves (Fig. 10.3). This means more information than received from CT GSN is used by hamsters to make behavioral discriminations among the tested taste stimuli. The CT contributes sucrose-sensitive (S) and NaCl-sensitive (N) CT GSN that are taste quality specialists, the former responding primarily to sweeteners (illustrated in the third column of recordings in Fig. 10.9b) and the latter responding to Na+ and Li+ halides (illustrated in the second column of recordings in Fig. 10.9b). T1R GPCR and ENaC ion channels (Fig. 10.6), their dedicated TC, and upstream S and N neurons form two peripheral lines to the brain that identify nutrients. In the CT stimulus space in Fig. 10.9c, note tightly clustered sweets and sodium salts falling distant from each other and a looser cluster for other stimuli. Responses to this broader cluster as well as the amiloride-insensitive response to NaCl (illustrated in the first column of recordings shown in Fig. 10.9b), occur in CT H GSN (also known as E GSN because of broad sensitivity to electrolytes). These more variable electrolyte-sensitive CT generalists respond to sour HCl and other acids, salty NaCl, bitter quinine and the bitter salts KCl and NH4Cl. Thus, each cluster in Fig. 10.9c corresponds to activation of the distinct hamster CT neuron types: the highly correlated responses of each of two types of specialists and loosely correlated responses of electrolyte generalists that are possibly composed of several more-specific subtypes.
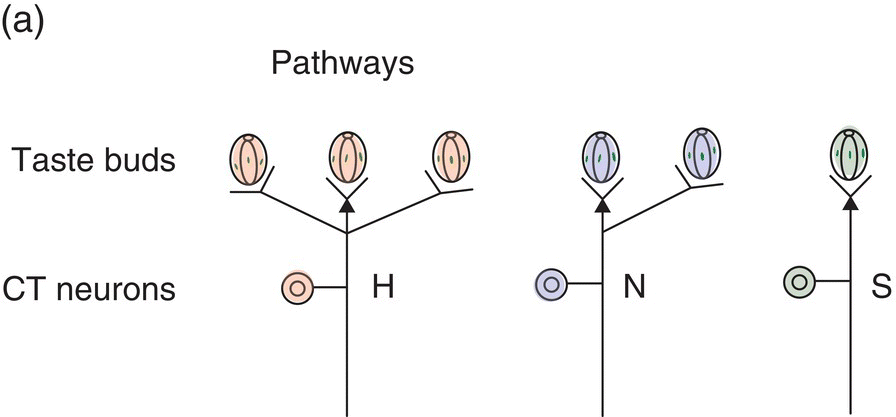
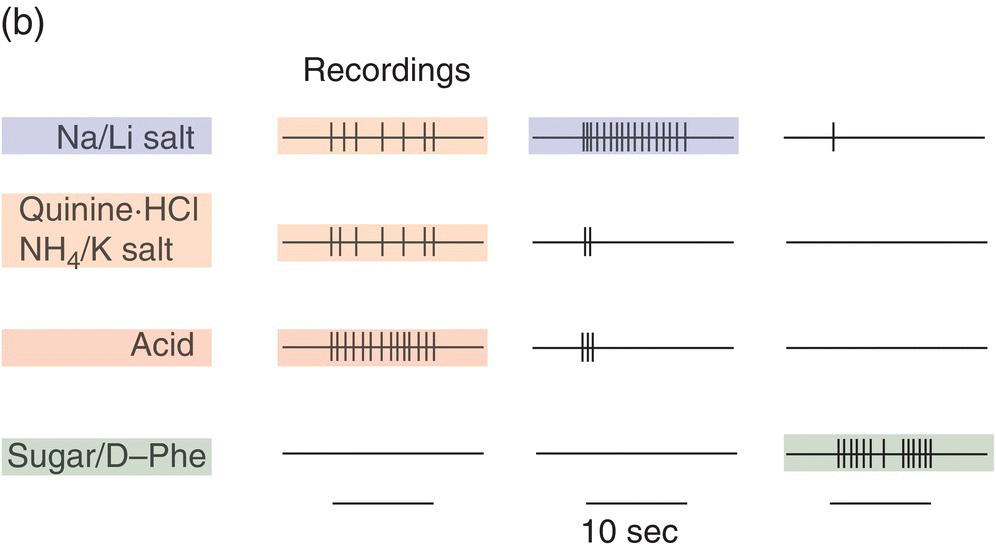
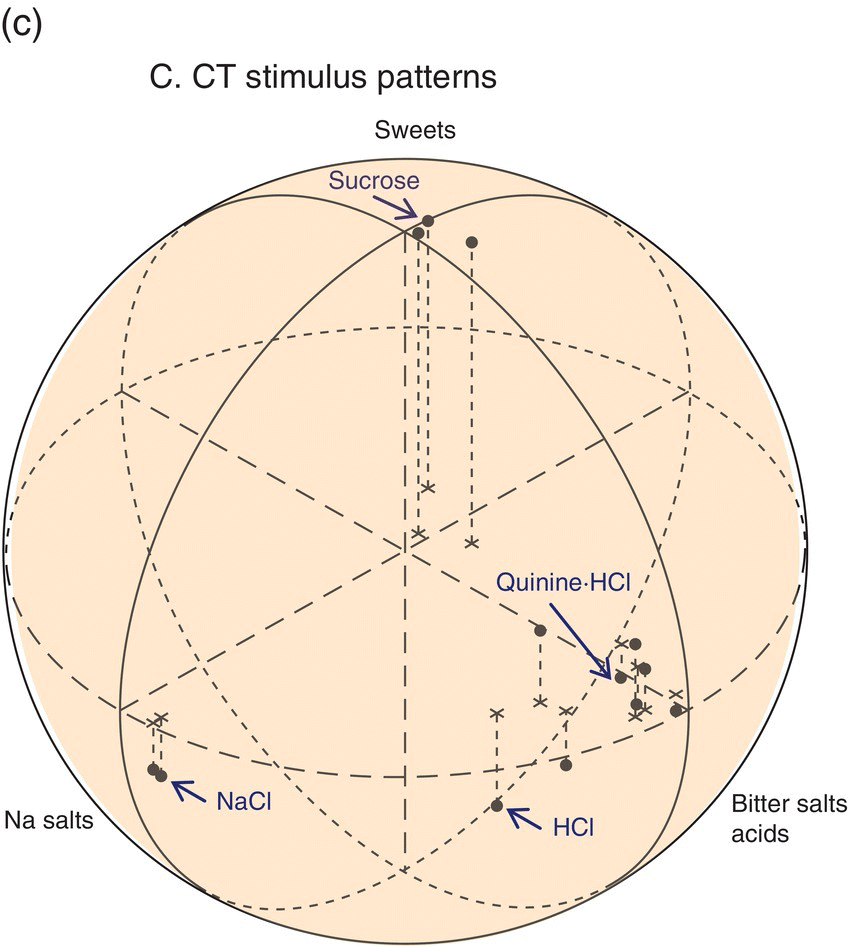
Figure 10.9 Electrophysiological responses of GSN single fibers in the chorda tympani (CT) nerve. Diagrams of (a) taste bud-to-GSN pathways for three physiological types of CT GSN (H, N, S) with (b) decisive single-neuron recordings and (c) a representation of neuronal stimulus patterns for sweets (sucrose, fructose, saccharin), sodium salts (NaCl, NaNO3), bitter salts (quinine⋅HCl, KCl, NH4Cl, MgSO4), urea, and acids (HCl, citric acid, acetic acid) in />
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


