9
Chronic Orofacial Pain: Biobehavioral Perspectives
- Defining pain: An unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.
- Biobehavioral perspectives on TMD: The most important chronic orofacial pain conditions dentists encounter.
- A biopsychosocial model for understanding chronic pain and its management
- The dentist as a biobehavioral clinician, applying a dual-axis approach to assess and manage the physical (Axis I) and emotional, behavioral, and psychosocial (Axis II) components of chronic orofacial pain.
Overview
Chronic pain in the masticatory muscles of the face and the temporomandibular joints, most commonly referred to as temporomandibular disorders (TMDs), but also known as temporomandibular joint disorder (TMJD), and historically by the less appropriate term, temporomandibular joint (TMJ), are by far the most prevalent of all chronic orofacial pain conditions. TMDs comprise a cluster of related chronic pain conditions that affect the hard and soft structures of the orofacial region characterized principally by (1) the presence of pain typically in the preauricular area in front of the ear, the cheeks, and/or temporal area; (2) limitations in movement of the mandible; and (3) joint sounds detected in the TMJ during functional excursions of the jaw.
TMD arises in an orofacial region which houses highly specialized organs of special sense, notably taste, smell, and hearing. In addition, dentistry is of course most familiar with the specialized structures of the oral cavity, especially the teeth, periodontium, oral mucosa, salivary glands, and tongue. Not surprisingly, this relatively compact anatomical region is not only highly vascularized but is further characterized by an especially complex network of central and autonomic nervous system pathways, including, especially, the second and third divisions of the trigeminal nerve. The craniofacial and perioral structures involved in TMD—the bones of the upper and lower jaw and the surrounding musculature—function as a stomatognathic system which is responsible for several life-sustaining physiologic processes, including eating, breathing, swallowing, and verbal as well as nonverbal communication. Inevitably, these physiologic processes are also associated with psychological and psychosocial functions of tremendous significance to the individual—functions that underlie the development of singularly unique intrapersonal and psychosocial characteristics distinguishing one person from another—and which affect how we look and how we express our meanings and our emotions.
Within this complex orofacial region, TMD emerges as a common chronic pain disorder, third in prevalence among common chronic pain problems according to the best available evidence, after low back pain and chronic headache. TMD is most prevalent in women during the reproductive years and falls off sharply with advancing middle age. It definitely appears in adolescence but is rare in older men. All epidemiologic studies of TMD report similar patterns (Drangsholt & LeResche, 1999). Other than the age and gender factors just mentioned, knowledge regarding other risk factors in adults for first-onset TMD are only just emerging and point to a complex intersection among clinical, health status, psychological, psychophysical, and genetic variables (Fillingim et al., 2013; Greenspan et al., 2013; Ohrbach et al., 2013; Sanders et al., 2013; Smith et al., forthcoming). Factors associated with the transition from acute pain to chronic pain are as yet unidentified.
While any one of the three major clinical indicators of TMD (i.e., pain, limitations in jaw opening, and joint noises) is estimated to be present in 5–50% of the population at any one time, treatment seeking seems only poorly correlated with the presence of TMD signs and symptoms other than pain. Intensity of TMD-related pain is the most reliable predictor of treatment seeking, and pain relief is the most important single criterion by which patients and clinicians alike judge the success of clinical treatment.
The scientific rationale for including TMD among important clinical chronic conditions rests on confirmed observations from scientific studies as well as abundant clinical experience that maladaptive overt behaviors, psychological status, and psychosocial functioning can all be impacted by persistent TMD-related pain. Chronic pain arising in conjunction with impairment of the masticatory system from whatever etiology impacts the lives of TMD pain sufferers—from mild alterations in eating behaviors to profoundly disabling depression and appreciable interference with activities of daily living. Indeed, all chronic pain conditions share features in common from the psychosocial domain (e.g., depression, limitations in activities, increased healthcare utilization), while each chronic pain subtype retains unique physical features from the biologic (or physical) domain related to specific body site (e.g., headache, back pain) or specific pathophysiologic processes (e.g., postherpetic neuralgia, cancer).
Chronic orofacial pain is always an organismic response, not simply a local phenomenon, because it always involves simultaneous processing of diverse types of information at different levels of integration. Pain is not a passive consequence of the transfer of a defined peripheral input, from say the masseter muscle, to only one center in the cortex. Thus, pain is an active process generated partly in the periphery and partly within the CNS. The available evidence suggests that the peripheral source of pain may be not only nociception, but it can also be based solely on nonnociceptive sensation which is thought to trigger CNS events that give rise directly to “pain.” Multiple plastic changes, of which the brain is especially capable of, result in, among other things, learning and memory and consequently determine the gain of the system (Woolf, 2007). The International Association for the Study of Pain (IASP) (Merskey & Bogduk, 1994) gold standard for the definition of pain and associated terms serves to reinforce this understanding:
Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage (p. 210). The IASP definition is further amplified (p. 210), as follows: Pain is unquestionably a sensation in a part or parts of the body … pain is always unpleasant and therefore, also an emotional experience … pain is always subjective and results from co-occurring factors including pathobiology, prior experience and social context.
Over the past six decades, tremendous advances have been made in identifying structures and processes—both physiologic and behavioral—which contribute to chronic pain phenomenology. These advances into domains either previously not even known to exist or not suspected of being implicated in the pain experience have been nothing less than breathtaking for the elegance of the science and the complexity of the phenomenology they reveal. Basic and transitional dental scientists as well as clinical dental researchers have made significant contributions to the overall advancement of our knowledge about pain, including chronic orofacial pain. Nevertheless, chronic TMD remains enigmatic, and in some quarters, highly controversial especially with regard to both unsubstantiated etiologic theories and unproven claims of treatment effectiveness.
In any event, it is entirely reasonable to maintain that TMD pains arise, fluctuate, persist, remit, and have the potential to be debilitating. Responsible scientists and clinicians, meanwhile, remain unable to integrate the diverse anatomic, physiologic, and psychologic data manifest in patient presentations into an etiologically based diagnostic system. Presently available diagnostic systems, such as the Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) and its recent successor, the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD), later discussed more fully, are based largely on reliable symptom descriptions and clinical examinations of presenting signs and symptoms.
The purpose of this chapter is to present current perspectives on assessment and treatment of chronic orofacial pain patients. These perspectives advocate understanding the chronic pain patient as an integration of objectively measured physical and pathologic findings with subjectively reported physical, psychological, and psychosocial conditions. The universally accepted model system for understanding chronic pain from these perspectives is labeled the biopsychosocial model for pain (Engel, 1977). Accordingly, the specific objectives of this chapter are to (1) define chronic pain and the most common chronic orofacial pain conditions confronting dentistry; (2) provide the rationale and description for the biopsychosocial model as applied to chronic orofacial pain; and (3) describe the role of the dentist as a biobehavioral clinician. In the context of this chapter, the clinical responsibilities of the dentist as a biobehavioral clinician extend to assessing the biobehavioral dimensions of chronic orofacial pain, which include psychological, psychosocial, and physical/pathologic assessments, and to integrating such biobehavioral assessment into evidence-based clinical decisions regarding the comprehensive management of the chronic orofacial pain patient.
Introduction
The most prevalent chronic orofacial pain conditions are musculoskeletal (e.g., TMD) and neuropathic (e.g., trigeminal neuralgia and atypical odontalgias) (LeResche, 2000). As a healthcare profession, dentistry is arguably unparalleled in reducing and eliminating acute pain (e.g., toothache, dento-alveolar, or periodontal abscess). However, chronic orofacial pain remains, unfortunately for too many patients, more resistant to quick or simple resolution because the amount and even the location of pain experienced and the behaviors of the patient with chronic or persistent pain problems are only poorly related to physical events, making etiology elusive and treatment difficult. Atypical odontalgia, for example, is usually associated with poorly defined pathologic markers inconsistent with expressed pain perception and behavior. Similarly, persistent pain in the masticatory muscles (myalgia) can be a source of minor inconvenience to some patients and for others can become a decades-long major disorganizing force associated with significant depression and disruption of their everyday lives—yet there may be no detectable, let alone diagnosable, physical change to distinguish the condition in these individuals. So, whether persistent pain is experienced as a minor inconvenience or as a source of major life disruption, chronic orofacial pain and related disability often cannot be understood in terms of diagnosable pathology (Dworkin, 1994).
Musculoskeletal pain, particularly TMD-related pain, is overwhelmingly the most commonly occurring chronic orofacial pain confronting dentistry, with a prevalence of about 10–15% in the United States and around the world (LeResche, 2000). TMD is associated with the same significant psychological and psychosocial issues that are found in all chronic pain conditions, orofacial or otherwise (Von Korff et al., 1988); much of the remainder of this chapter uses TMD-related pain to elucidate major concepts (see Suvinen et al., 2005, for a comprehensive review) as well as methods for biobehavioral assessment and management.
Toward a Biopsychosocial Model for Chronic Orofacial Pain
Newly emerging fields of brain-behavior neuroscience—including the use of functional MRI and other brain-imaging modalities to visualize brain responses in real time—and the rapidly emerging field of epigenetics—demonstrating how the environment influences gene expression—has provided new insights into the biologic basis for subjective experience. From these exciting new fields emerges a science-based rationale for understanding how emotional, cognitive, and behavioral processes can become linked and stored as integrated neural circuits, or neuromatrices, preserving memories and belief systems which influence, among other things, our subjective pain experience and how we come to the behaviors we take to cope with pain.
These complex biologic interactions reflecting the multiple dimensions of pain are understood to be under the influence of such objective biologic factors as, for example, genes (Mogil, Seltzer, & Devor, 2004) and reproductive hormonal factors (LeResche et al., 1997). Each individual learns about pain through experiences related to injury in early life. Biologists recognize that those stimuli which cause pain are liable to damage tissue. Accordingly, pain is always a subjective experience we associate with actual or potential tissue damage (Merskey & Bogduk, 1994). It is unquestionably a sensation in a part or parts of the body, but it is also always unpleasant and therefore also an emotional experience. Alternatively, activity induced in the nociceptor and nociceptive pathways by a noxious stimulus, in the absence of the subjective self-report of the aversive experience, is not pain. In fact, finding neural activity in nociceptors and pathways known to conduct painful impulse is referred to specifically as nociception (but not pain), in contrast to pain which is, as the IASP definition so clearly states, a subjective experience.
Conversely, patients often report pain in the absence of detectable tissue damage or pathophysiological cause. Such subjective pain reports are always best presumed an accurate reflection of the patient’s experience even if the dentist cannot find an objective biologic explanation for the pain symptom. It has been historically the case that pain symptoms not accompanied by a detectable physical basis for the pain have, unfortunately, led well-meaning clinical practitioners—both dentists and physicians—to attribute the pain report to psychological rather than organic etiology, implying that somehow the pain is imagined, hence, not “real,” We find such an approach of little practical value for chronic pain because there is usually no way to distinguish the physical but undetected (and perhaps undetectable) cause, versus higher-order brain mechanisms that clearly are able to allow pain to be experienced in the clear absence of any physical basis (e.g., phantom limb pain, where pain is reported in a limb which has been amputated). If the patient regards their experience as pain and reports it in the same way as pain caused by tissue damage, it should be accepted as pain and treated accordingly. This definition avoids tying pain exclusively to an identifiable peripheral stimulus and avoids the potential pitfall of attributing a patient’s subjective report as reflective of either real or imagined pain. Instead, a more useful (and far more palatable for patients) view is that pain report in the absence of discernible tissue damage can potentially arise from multiple reasons (e.g., physical, psychological, social, and/or cultural processes) that invoke physiologic activity that can be experienced subjectively as pain.
The Biopsychosocial Model
Mechanistic, biomedical views of pain as originating in the body and arising or maintained solely from objectively measurable pathophysiology are now considered scientifically inadequate to fully explain a chronic pain patient’s presentation. It is now understood that healthcare providers have an inherent need (if not ethical mandate) to rely on the patient as the only reliable source for knowing whether or not pain is present. We simultaneously recognize that the subjective pain report is the result of many co-occurring factors, ranging from pathobiology to prior experience to social context. Hence, the biomedical model of pain has been outgrown because of its too exclusive emphasis on physical factors and observable tissue damage. The biomedical model of pain has been succeeded by a biopsychosocial model system (discussed more fully in a later discussion) which clarifies how physical events in the body or in the environment often can give rise to not readily predicted pain experiences and behaviors that can be dependably shown to be related to patients’ past pain history, gender, ethnicity, and, most critical for dentistry, factors in the environment which are equated with pain or the potential for pain. The term biopsychosocial has been applied to the most widely used model system developed to capture how information processing of painful events implicates psychological, behavioral, and psychosocial factors that interact with the physical events. The resultant of this complex interactive process is the wide range of pain expression which dentists struggle to incorporate into their overall approach to management of dental and orofacial pain.
Figure 9.1 presents, in schematic form, a model for integrating presumed physiologic activity associated with ongoing pain experience. The model depicts that successively higher levels of central processing by the brain integrate the nociceptive or harmful stimuli present in the pain transmission system and underlie the emergence in consciousness as pain. Higher-order central processing assigns meaning to the pain for each individual, which then mobilizes patients to act in response to their uniquely defined pain state and within the social context of permissible behaviors. Because the model system seeks to integrate physiologic or pathophysiologic activity with associated psychological states and socially and culturally determined behavior, the model is labeled a bio-psycho-social model—that is, a model integrating biologic, psychologic, and social components of the pain experience (Dworkin, Von Korff, & LeResche, 1992).
Fig. 9.1. Biopsychosocial model for pain. The model’s five-stage processes integrate physiologic or pathophysiologic activity with associated psychological states and socially and culturally determined behavior.
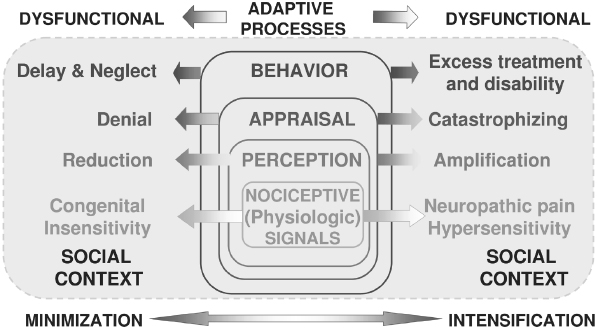
The stages of the pain experience offered by this model system all reflect normal coping mechanisms by which individuals come to experience pain and then attempt to make sense of the pain and adapt appropriately. These same higher-order processes are subject to distortions and maladaptive responses as well, and some examples are given for each level at which it is possible to analyze complex expressions of pain using this biopsychosocial model:
- Nociception: Physiologic events associated with actual or potential tissue damage that, among other things, provides information via the pain transmission system sensory information to higher centers dealing with attention, memory, emotions, decision-making, and motor preparedness.
- Perception: The initial stage of forming a subjective pain response, self-identifying the physical qualities of the pain experience, which include sensory (e.g., sharp, dull, throbbing), spatial (e.g., highly localized to an specific anatomic site, as in acute toothache, or diffuse, as in many cases of TMD pain), and temporal (e.g., acutely arising, recent onset, as in toothache, or recurrent and persistent over time, as in chronic TMD pain qualities
- Appraisal: Higher-order integrative mental operations attaching cognitive and emotional meaning to the painful sensations being perceived. The appraisal level is crucial for attaching attitudes, beliefs, expectations, and emotional arousal to those pain sensations—in a word, meaning is attributed to the physical experience. Inappropriate attribution of meaning, influenced by interaction of nociceptive activity with attention and memory, may yield cognitive processes and emotional states which present as pain-related catastrophizing thoughts, fear, anxiety, or depression.
- Behavior: Observable pain behaviors that are either contributory (e.g., bruxism) or the result of pain (verbal and nonverbal expressions of pain, inactivity, diet modification). Fordyce’s introduction of the notion of ‘chronic pain behavior’ into the rehabilitation of chronic pain patients was a revolutionary concept that called attention to the possibility for chronic pain conditions to become associated with maladaptive behavioral patterns of work or social avoidance (Fordyce, 1976). The implication of this notion is that chronic pain treatment should not focus exclusively on uncovering difficult-to-observe pathophysiology but should focus heavily on behaviorally based methods for returning the pain patient to a more productive lifestyle. In more recent years, many of Fordyce’s seminal concepts, clearly anchored in reinforcement models associated with learning theory, have reemerged in the fear-avoidance model (Vlaeyen & Linton, 2000). The fear-avoidance model—sometimes called the fear of (re)injury model—is relevant to musculoskeletal pain in particular, and it indicates that dysfunctional beliefs regarding the nature of an injury or a pain experience lead to behavioral adaptations in the individual’s behavior which ultimately lead to an increasingly more restricted scope of behavioral range (e.g., avoiding use of the jaw for anything other than talking through clenched teeth) and deconditioning, such that any attempt at normal use immediately results in sensations perceived as more disabling pain and identified by the individual as evidence of further tissue damage. One of the salient characteristics of the fear-avoidance model is that it well explains a series of events, from tissue damage to ostensible healing accompanied by persistent pain; incorporates depression and disability; and leads to behaviorally oriented therapies.
- Sick Role: Cultural and societal factors shape the pain experience by defining roles for pain patients that may sanction disability as well as lead to specific forms of pain-related health care and medications. Sanctioning of different sick roles for men and women in response to pain is an important example of the influence that social factors can play in determining observable manifestations of pain. Options for treatment of dental and orofacial pain are often constrained by factors dictated by social or cultural factors, such as availability of health insurance and governmental regulation of narcotic analgesics. However, the sick role for a significant minority of patients experiencing chronic pain is associated with heavy use of healthcare services and demand for narcotic pain medications, both examples of pain behaviors that can run counter to social norms in many parts of the world. Conversely, a patient may inappropriately reject the sick role and consequently avoid treatment that could be beneficial.
In summary, the biopsychosocial model, as its name implies, reflects our growing understanding that illnesses and cures are indeed complex. In order to understand how and when we experience pain and to understand whether or not we will respond to treatment, a host of factors in addition to biology must be considered. The biopsychosocial model does not seek to compete with, let alone replace, scientifically derived biologic models or current clinical practices. Rather, the model is an integrative one, which conceives that biologic processes and environmental factors, in the broad sense defined earlier, are equipotent for explaining not only pain conditions but also responses to treatment for the alleviation of pain. Moreover, the model indicates that the provider must start from a view of inclusiveness b/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


