Failure in Osseointegration
Kumar C. Shah1, S. Andrew Chapokas2, and Sreenivas Koka3
1 University of California Los Angeles School of Dentistry, Los Angeles, California, USA
2 San Diego, California, USA
3 Loma Linda University School of Dentistry, Loma Linda, California, USA
Introduction
Successful osseointegration is the result of a state of “osseosufficiency”, where the net contribution of the patient (host), the implant, and the clinician combine to promote and perpetuate osseointegration.1 Following on, when an insufficient state results, the interface between host bone and an implant deteriorates and experiences “osseoseparation” (Table 8.1). In this chapter, we discuss the roles of patient, implant, and clinician as they relate to osseosufficiency, osseoseparation, and failure of osseointegration. In particular, we challenge the dogma that “implants fail” and ask all clinician scholars to ask themselves, “Do implants really fail?” in the way they are purported to behave. Or, in reality, are implants “retrieved”, normally as a consequence of a combined set of events far more dependent upon the behavior of the patient and the clinician than of the implant? Perhaps our desire to find someone or something to blame when an implant is retrieved predisposes us to blame the one element that cannot answer back – the implant. In this chapter, we discuss in detail key attributes of patients, clinicians, and implants that are associated with failure in osseointegration.
Table 8.1 Osseoseparation staging system.
| Stage 0 osseoseparation |
| Implant is asymptomatic and immobile “Nuisance gingivitis” may or may not be present Marginal bone loss is minimal Eligible for prosthodontic abutment service with a predictable esthetic outcome |
| Stage I osseoseparation |
| Implant is asymptomatic and immobile Gingivitis may or may not be present Eligible for prosthodontic abutment service, although marginal bone loss and minimal accompanying implant material exposure may preclude a satisfactory esthetic outcome Readily managed with routine debridement and oral hygiene protocols Additional clinical intervention is not required because of noncompromising site location, e.g., favorable circumoral lip morphology. Alternatively, minor prosthetic design alterations may be indicated |
| Stage II osseoseparation |
| Implant is asymptomatic and immobile Gingivitis is often present Frequently precludes esthetically satisfactory prosthodontic outcome since the amount of marginal bone loss also exposes implant threads Favorable oral hygiene considerations or a satisfactory esthetic outcome cannot be achieved without secondary changes in prosthesis design or attempting secondary plastic gingival surgical interventions |
| Stage III osseoseparation |
| Bone loss location may be variable and often substantial, although the implant(s) remain(s) asymptomatic and immobile Gingivitis almost invariably present Only eligible for prosthodontic abutment service if resultant esthetic and oral hygiene concerns can be rectified by additional interventions Treatment outcome and prognostic considerations (both patient‐ and dentist‐mediated ones) frequently demand change(s) in the original treatment plan, including removal of the implant(s) and surgical site development |
| Stage IV osseoseparation |
| Implant is usually symptomatic and slight mobility is almost invariably present Bone loss may present as either inconsequential or substantially so Gingivitis may or may not be present Biologic failure of the osseointegration process has occurred (early failure) or has developed gradually on an osseoinsufficiency/time‐dependent basis (late failure) |
Patient factors
In order for dental implants to remain “healthy” and in function, it is clear that direct bone apposition is required with minimally inflamed soft tissue surrounding the transmucosal interface. Although a certain amount of marginal bone loss may be tolerated, progression of bone loss may pose a serious threat to implant longevity. Criteria for implant success have been defined with a need for a reliable presence of bone around dental implants.2 More recently, the previous nomenclature for peri‐implant pathology has been challenged. Traditionally, inflammation with crestal bone loss around dental implants has been termed peri‐implantitis. When gingival and/or mucosal inflammation exists, without bone loss, the term peri‐implant mucositis is used. As the mechanisms for peri‐implant pathology have been evolving, application of new terminology has been suggested.1,3 Osseosufficiency best describes the process whereby osseointegration is maintained. When the soft tissue and bone–implant interfaces are compromised, osseoseparation has been used as a replacement of the term peri‐implantitis.4 Differentiation between primary and secondary peri‐implantitis has been offered to clarify whether or not infection was considered as the primary cause for peri‐implant bone loss or not, respectively.3 Regardless of the terms used, new insight to peri‐implant disease will be described that may entirely change the way that we understand the processes of developing, maintained, and lost osseointegration.
Several risk factors for peri‐implantitis and marginal bone loss have been proposed, and the primary theories have been classically considered to be infection or overloading of dental implants. However, it is controversial as to which mechanisms best explain changes in the inflammatory status and marginal bone loss around dental implants. Until recently, biomaterials, surgical hardware, and any other foreign material have been understood as inert bodies that have no specific effect on the immune system and/or surrounding tissues. Now, there has been a paradigm shift in the approach to investigating the causes of peri‐implantitis and associated peri‐implant marginal bone loss. Osseointegration is better understood as a foreign body equilibrium reaction that requires a series of complex mechanisms in order to avoid host rejection that may manifest as peri‐implant marginal bone loss and soft tissue inflammation (Figure 8.1). Therefore, the immune system dynamically determines the fate of any implant within living tissues.5,6 In order to understand osseointegration, both the biomaterials and host immune characteristics need to be addressed.
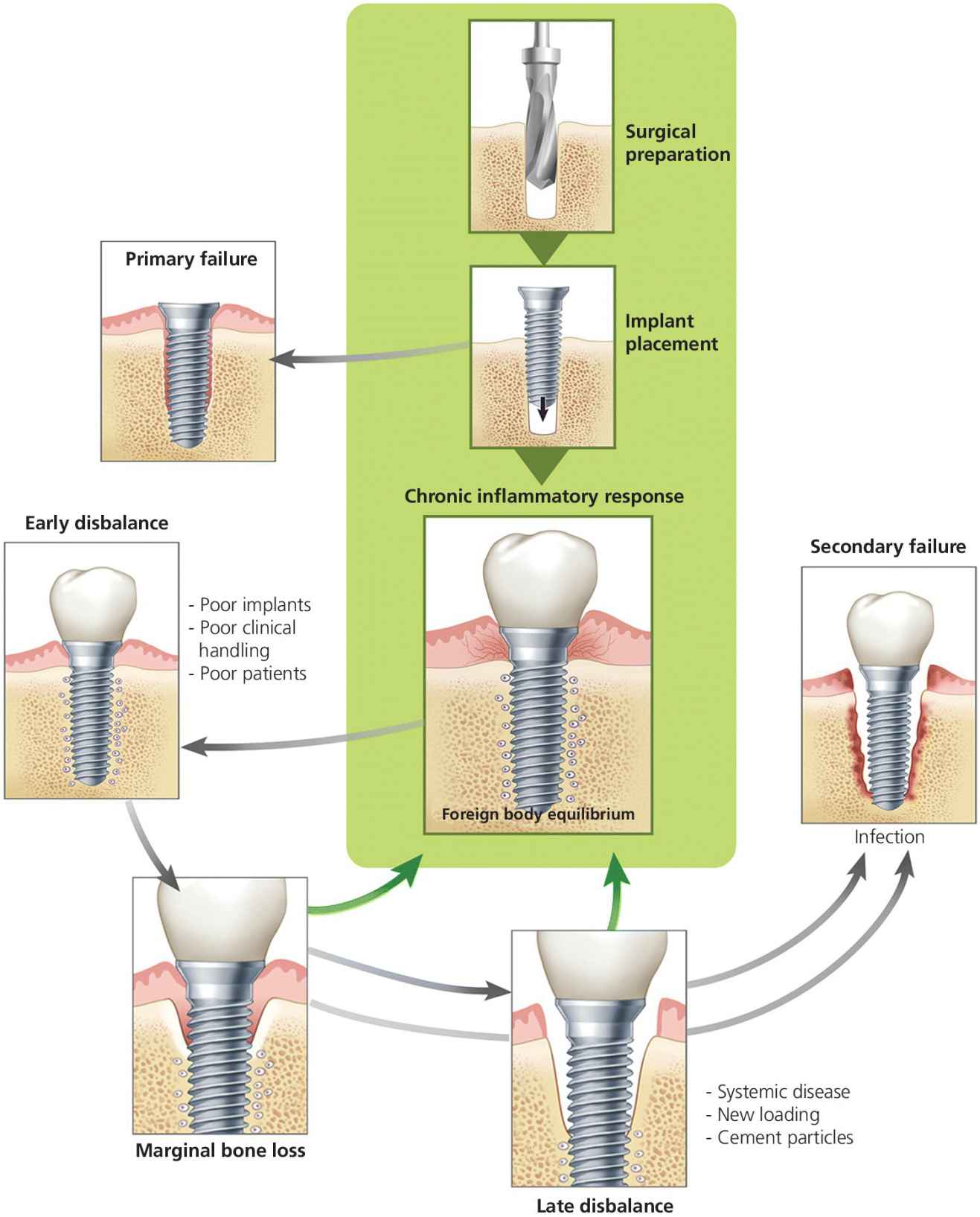
Figure 8.1 Foreign body equilibrium reaction.
Source: © 2016, Chris Gralapp.
Foreign body reactions are characterized by granulomatous inflammation, which is a distinctive pattern of chronic inflammation that occurs for a limited number of infectious and noninfectious conditions. When a certain offender is difficult or impossible to eradicate, a granuloma will typically form in attempt to contain it. As in other types of chronic inflammation, tissue injury and attempted repair coexist over long or indefinite periods of time. Macrophages are the dominant cell type in chronic inflammation, and are differentiated from the mononuclear phagocyte system.7 It is widely understood that macrophages play a key role this process and have been stratified into two different phenotypes. M1 macrophages are reactive, phagocytic, and proinflammatory, whereas M2 macrophages are more involved in the simultaneous repair phases that occur during chronic inflammation.8
Blood monocytes traffic into connective tissue or certain organs such as the liver (Kupffer cells) and central nervous system (microglia). Furthermore, bone‐specific macrophages called osteomacs, have been identified on or near periosteal surfaces forming a canopy‐like structure over mature osteoblasts. Although it is unclear whether osteomacs play a role in bone remodeling, newer emerging data suggest that osteomac populations play a role in bone homeostasis. Osteomac expression of cytokines, such as transforming growth factor (TGF)‐beta and ephrin B2, provide coupling signals that have been previously implicated in osteoclastic regulation of bone formation.9
Granulomatous reactions are categorized based on two different types of pathogenic responses. Foreign body granulomas develop in response to inert foreign bodies. Histologically, macrophages aggregate, transform into epithelial‐like cells, and may later fuse to become multinucleated giant cells (Langerhans or foreign body type with no known functional difference at this time). Typically, the foreign body will be in the center of the granuloma, does not incite any specific immune response, and is too large to allow phagocytosis by macrophages. At the periphery of the granuloma, there is a layer of mononuclear leukocytes, consisting of mostly lymphocytes and occasionally plasma cells. On the other hand, immune granulomas are caused by agents that can induce a cell‐mediated immune response. A more complex series of immunogenic reactions takes place whereby macrophages ingest foreign protein antigen and present it to antigen‐specific T lymphocytes. This process results in a series of cellular interactions through the production of several proinflammatory cytokines, such as interleukin (IL)‐2 and interferon (IFN)‐gamma that ultimately increase the transformation of macrophages to epithelioid cells and multinucleated giant cells. The most common example of an immune granuloma occurs in association with Mycobacterium tuberculosis.7
Until recently, dental implants have only been considered as inert biomaterials having no specific immunological effects. It now seems that the contrary is the case. Histologic evidence demonstrates the presence of multinucleated giant cells regularly found at the bone–titanium interface. Even though this alone can justify the concept of osseointegration being a foreign body reaction, surface protein adsorption by titanium and other biomaterials has been demonstrated to have significant effects on the host response to further this concept. Certain proteins, such as fibrinogen, have been demonstrated to have hidden amino acid sequences that become exposed when adsorbed to a surface and/or denatured when compared to the soluble form.10 When proteins are absorbed, they are unfolded and different antigenic sequences are exposed with the capability of inducing an entire host of molecular and cellular responses. The net result of these reactions can naturally have negative and/or positive effects.
Considering osseointegration as a dynamic series of complex host immune interactions, Albrektsson and colleagues6 propose the following: 1) osseointegration is the result of a foreign body reaction that, with the right intensity in the inflammatory response, will balance itself out and allow for bone to grow on the implant surface; 2) similar to soft‐tissue implants, which end up in poorly innervated and vascularized fibrous tissue, dental implants also become surrounded by condensed bone that is very poor in vascularization and innervation typical of a foreign body reaction that has reached equilibrium; and 3) this newly formed bone may be seen as a manner of shielding off the foreign entity from the tissues as a protective mechanism.2
Then, once an implant has “successfully” integrated, why does implant retrieval occur? What is the most plausible evidence? As mentioned previously, up until this time, infection and overloading have been the most accepted theories. However, there is no evidence of a primarily infection‐driven reason for marginal bone loss around dental implants.3 Some data exist to suggest that overloading of dental implants may result in marginal bone loss, but there is no evidence that suggests this is the sole factor when it is involved. There is most likely a disturbance in the foreign body equilibrium that changes the immunologic nature of the surrounding cells, resulting in marginal bone loss that is secondarily colonized by bacterial offenders.5 This complex series of interactions can be initiated by a variety of biologic responses, including different dendritic cell phenotypes, autoimmune disorders, influence of medication, such as selective serotonin reuptake inhibitors (SSRIs), and even additional aseptic foreign body reactions to surrounding cement particles.6,11–13 Further investigation and understanding of phenotypical variation in patients may provide better insight into the critical role of the immune system with regards to the fate of an implant in both the short and long term.
Implant factors
The dental literature exhaustively describes implant design variables that may influence osseointegration. Implant manufacturers have presented anecdotal clinical evidence suggesting clinical success with their implant systems. We will review some implant design variables and discuss how implant therapy is influenced by these variables.
Implant surface/coating
Since the introduction of the machined Branemark implant, multiple different roughened or micro‐roughened implant surfaces have been introduced. For successful osseointegration, the biologic processes of blood clot formation, release of growth factors, and osteogenesis are paramount. A roughened surface improves the surface tension and wettability of the surface to plasma proteins. Consequently, platelets and growth factors from the fibrin clot attract migratory osteoprogenitor cells. Osteoprogenitor cells in the wound site initially deposit woven bone. Davies14,15 described distance and contact osteogenesis, where, in distance osteogenesis, new bone is formed on the surface of old bone away from the implant surface, and contact osteogenesis is bone formation by those cells on the implant surface. When comparing distant and contact osteogenesis, implant surface characteristics preferentially influence contact osteogenesis.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


