Chapter 7 Orofacial bacterial infections
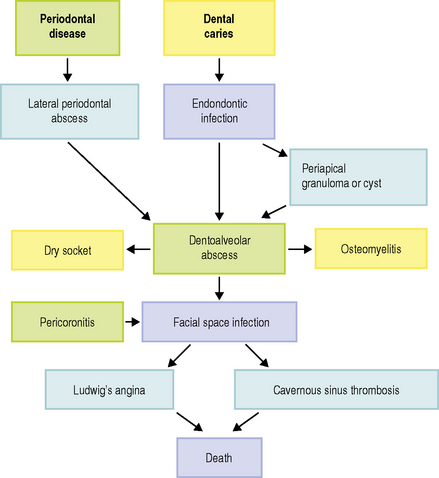
As described in previous chapters, the mouth contains a rich and diverse microflora, only 50% of which can be cultivated. Dental plaque within a healthy mouth is a complex biofilm (Ch. 5), which has a commensal relationship with the host. However, plaque can become ‘pathogenic’ since it is involved in the two most prevalent human diseases, namely dental caries and periodontal disease (Ch. 6). Untreated, both of these conditions can progress into other forms of acute and chronic infection within the mouth and orofacial tissues (Fig 7.1). Small alterations in the local environment can produce microbial population changes that permit the development of opportunistic infections involving bacterial species that are usually regarded as non-pathogenic members of the oral microflora. On occasions, these infections can produce severe life-threatening situations.
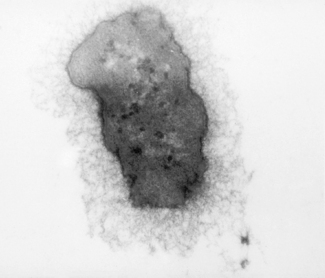
Contemporary microbiological studies have revealed that the types of bacteria recovered from orofacial dental infections reflect the wide spectrum of facultative and strictly anaerobic bacteria that also are regarded as indigenous constituents of the host’s oral microflora. Strict anaerobes comprise a major proportion of the microflora within acute suppurative infections. In addition, pathogenicity experiments in animals have implicated strictly anaerobic bacterial species, in particular Gram negative bacilli, as not only the predominant species but also the most likely pathogens, although the reasons for this are uncertain. One possibility is the occurrence of specific combinations of bacterial species since animal models have shown that Prevotella spp and Fusobacterium spp are more pathogenic when in combination with members of the anginosus group of streptococci than when inoculated subcutaneously separately (pathogenic synergism; Ch. 6). It has also been proposed that microaerophilic species produce an environment that favours the proliferation of strictly anaerobic species. The presence of an extracellular capsule on certain bacterial strains recovered from dentoalveolar abscesses has also been implicated as a potential pathogenic determinant since this may protect bacteria from phagocytosis or intracellular killing (Fig. 7.2). While found on fresh clinical isolates, the capsule is lost after repeated subculture in vitro.
In an infection involving a diverse microbial community it is likely that environmental factors, such as availability of nutrients, local pH and the status of the immune defences, also play a major contributing role in determining the clinical outcome and whether or not an acute suppurative process develops. The primary source of nutrients is serum-derived proteins along with some host tissue components. Bacterial species within the infection may produce a range of complementary enzymes, in particular glycosidases and proteases, that permit progression of the infection (Ch. 4). Prevotella oralis, P. intermedia and Porphyromonas endodontalis have been identified as particularly effective in the degradation of serum proteins and immunoglobulins. These species also obtain essential growth elements such as iron and haemin from the catabolism of albumin, haptoglobin, haemopexin and transferrin. An example of the inter-relationship among the bacteria within the polymicrobial community is the degradation of proteins and peptides by Prevotella spp. for bacteria such as F. nucleatum, Eubacterium spp. and anaerobic streptococci. These consortia also produce metabolic substances, in particular hydrogen sulphide, indoles and amines, that inactivate host polymorphonuclear leukocytes and prevent complement action, in addition to acidic end products of metabolism that are cytotoxic.
Laboratory diagnosis
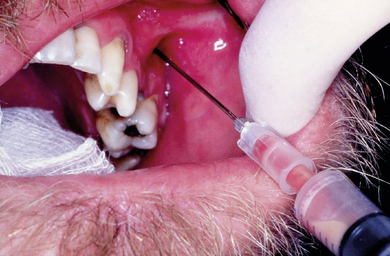
A major problem associated with the recovery of the causative microorganisms from specific orofacial infections is the high potential for sample contamination from microorganisms present in saliva. Contamination of a specimen with relatively rapid growing bacteria, such as streptococci or staphylococci, can potentially prevent the isolation of more slowly growing relevant species. As a basic principle the eventual microbiological report can only be as good as the quality of the specimen. In view of the likely presence of oxygen-sensitive bacteria in orofacial infections, all efforts must be made to ensure successful recovery of such strict anaerobes. Samples of pus should be obtained by aspiration to minimize the risk of contamination and protect oxygen-sensitive anaerobes from atmospheric oxygen (Fig. 7.3). If a swab is the only option for sampling, then this should be placed in appropriate transport medium. A microbiological specimen is a ‘living’ sample and as such must be transferred to the laboratory as rapidly as possible for processing to minimise the loss of viable bacteria.
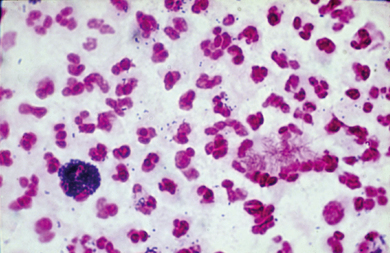
On arrival at the laboratory a Gram stain of a smear of the sample can be used to confirm that the specimen is truly pus and not another substance, such as cyst fluid. The Gram stain of pus will reveal a large number of polymorphonuclear leukocytes and bacteria, probably a mixture of Gram positive and Gram negative bacteria (Fig. 7.4). The sample will routinely be plated onto non-selective blood-based media which will be incubated in an atmosphere of air plus 5% CO2 and anaerobically. Fastidious anaerobe agar is also used to ensure isolation of strict anaerobes. Plates are incubated at 37 °C and examined after 18–24 hours for primary growth before being returned to the incubator for prolonged incubation and re-examination on a daily basis up to 7 days. Representative colonies of any detected growth are subcultured for pure growth and determination of atmospheric requirements. Identification of bacteria within orofacial infections can take a number of days due to the slow-growing nature of many strict anaerobes. This factor limits the clinical benefit of sampling such infections. More recently, molecular-based techniques have been developed to provide rapid identification and detection of specific bacterial species in dental infections (Chs 3 and 4). Rapid identification methods will improve the clinical usefulness of microbiology when managing severe dental infections.
Culture methods fail to recover the full diversity of microorganisms within orofacial infections (Ch. 3). Molecular studies, in which DNA has been extracted from pus obtained from acute dentoalveolar abscesses and 16S rDNA has been amplified using universal primers, have revealed that unculturable species account for a high percentage of the microflora in the sample when compared to results of culture. Similar findings have recently been reported for endodontic infections. These observations should be taken into account when considering the microbiological aspects of the individual infections described below since it is likely that unculturable and novel bacterial species have an aetiological role in all dental infections but their exact role is not fully understood at the present time.
Susceptibility testing is traditionally performed using a disc diffusion method on solid agar media. This technique allows a basic assessment of susceptibility. Calculation of the minimum inhibitory concentration (MIC) requires more labour intensive broth or agar dilution methods. In recent years, the development of a simple disc diffusion method called the E-test has permitted direct reading of antimicrobial MIC from an agar plate (Fig. 7.5).
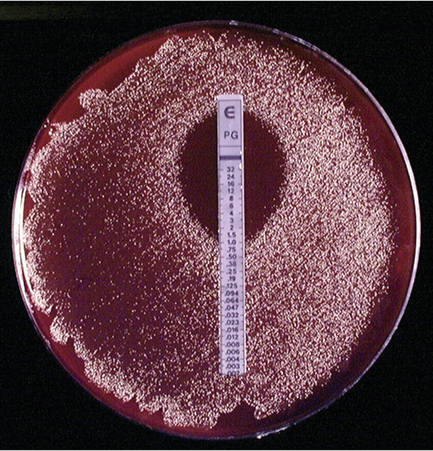
Fig. 7.5 Incubated plate of a Prevotella species demonstrating susceptibility to penicillin using the E-test.
Molecular techniques have been developed that permit rapid detection of penicillin-resistance genes in pus specimens. These techniques may prove to be helpful in clinical management if they become more widely available in the future. The presence of penicillin-resistant bacteria has been reported to be responsible for treatment failures in head and neck infections of dental origin. The production of beta-lactamases not only plays a direct pathogenic role by destroying the drug but also indirectly ‘shields’ non-beta-lactamase producing, penicillin-sensitive bacteria within the infection. The widespread use of penicillin has contributed to this problem, because it has been shown that the administration of penicillin leads to the emergence of beta-lactamase-producing bacteria, especially Gram negative bacilli, in sites such as the oropharynx. Increased resistance to other antibiotics prescribed for dental infections does not appear to have been reported. Of specific interest is the extremely low incidence of resistance to clindamycin, even in countries such as Germany and Japan, where this agent is frequently used to treat acute dental infections.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses