Chapter 7
Liner and bases
DEFINITION
Intermediate restorative materials are materials that are placed between a restoration and the dentine with a primary function of protecting the pulp. Dental liners, bases and cavity varnishes are used for this purpose.
A dental liner is a material that is usually placed in a thin layer over exposed dentine within a cavity preparation. Its functions are dentinal sealing, pulpal protection, thermal insulation and stimulation of the formation of irregular secondary (tertiary) dentine.
A dental base is a material that is placed on the floor of the cavity preparation in a relatively thick layer. Its purpose is to protect the pulp by providing thermal insulation due to temperature changes and absorbing occlusal forces. It can also be used to line out undercut areas for indirect restorations such as gold or composite inlays.
Cavity varnishes are used to seal the dentinal tubules in order to reduce microleakage that may cause sensitivity, discoloration and bacterial invasion. Dental varnishes are indicated under amalgam restorations and prevent moisture contamination in newly placed glass ionomer restorations. (Cavity varnishes have been largely replaced by dentine bonding agents). However it is not compatible with resin composite materials because it blocks adhesion and has a detrimental effect on the bonding properties. Cavity varnish may be used to protect the external surface of a newly placed glass ionomer cement restoration from early moisture contamination.
Bases and liners are ‘sandwiched’ between the cavity preparation and the restorative material of the operator’s choice; they may be referred to as intermediary materials. Although the function of a base and a liner is pulpal protection, the way this is achieved varies depending on the properties of the materials.
| Dental liner | Dental base | Cavity varnish |
| Glass ionomer | Zinc oxide eugenol | Copal varnish |
| Calcium hydroxide | Zinc phosphate | |
| Glass ionomer | ||
| Polycarboxylate | ||
| Flowable resin |
CALCIUM HYDROXIDE
Calcium hydroxide is a dental liner that stimulates the formation of irregular secondary (tertiary) dentine. It also has a function for direct or indirect pulp capping (see Chapter 9), but will only be discussed in this chapter as a dental liner .
Material constituents/composition
Most often supplied in a two-paste system – ‘catalyst’ and ‘base’. Constituents vary between manufacturers. The below constituents are specific to Life (Kerr).
| Catalyst | Base |
| Barium sulphate | Calcium hydroxide |
| Titanium dioxide | Zinc oxide |
| Methyl salicylate | Butyl benzene sulfonamide |
Calcium hydroxide is the active ingredient in the material, and the other ingredients may vary depending on the manufacturer.
Calcium hydroxide may also be supplied in a light-cured form
| Light-cured form |
| Urethane dimethacrylate resin (shrinks on setting) |
| Calcium hydroxide |
| Barium sulphate fillers |
Properties
- Low thermal conductivity
- Stimulates the production of irregular secondary (tertiary) dentine
- pH of 11–12 (i.e. alkaline)
- Bactericidal properties
- Highly soluble (not applicable to resin version)
Advantages
- Easily manipulated
- Stimulates the formation of irregular secondary (tertiary) dentine
Disadvantages
- Moisture sensitive
- Low strength
Indications and contraindications for use
Indications
- Used in the deepest portion of cavity preparation
- For use with direct or indirect pulp capping
- Only used when within 1–2 mm of pulp or direct pulp capping
- May be used underneath a base
Contraindications
- Cannot be applied thick enough to provide thermal protection for the pulp due to poor strength
Trade names
| Trade name | Manufacturer |
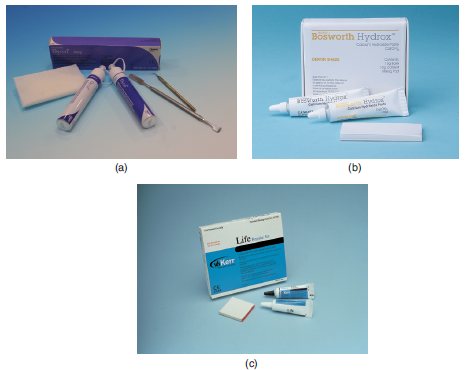
| Dycal® (Figure 7.1a) | Dentsply |
| HydroxTM (Figure 7.1b) | Bosworth |
| Life (Figure 7.1c) | Kerr |
| VLC Dycal® | Dentsply |
Figure 7.1 (a) Dycal® – Dentsply. (b) HydroxTM – Bosworth. (c) Life – Kerr.
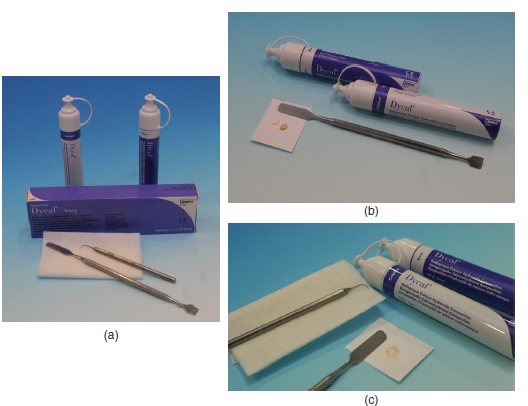
Manipulation (Figures 7.2a–7.2c)
Wearing personal protective equipment:
Two-paste system
- Dispense equal volumes of both catalyst and base onto a waxed paper pad according to the manufacturer’s instructions (use sparingly as not much material is needed)
- Do not allow the two pastes to touch
- Ensure that the correct caps are replaced on the appropriate tube to prevent cross-contamination of the base and catalyst
- Mix pastes together in a circular motion until a homogeneous (even) colour is achieved (10–15 seconds)
- Pass the material to the operator along with a calcium hydroxide applicator for application
- Have some gauze at hand to wipe the calcium hydroxide instrument in between applications
- Receive the calcium hydroxide instrument from the operator
- Wipe excess material from the spatula
- Dispose of the waxed paper pad and excess material in the contaminated waste bin
Light-cured system
- Dispense material
- Pass material (usually supplied in syringe form) to the operator
- Receive the calcium hydroxide syringe from the operator
- Light cure material as per manufacturer’s instructions
- Wipe excess material from the calcium hydroxide instrument
- Dispose of capsule and/or waxed paper pad in the contaminated waste
- Two-paste system – 10–15 seconds, light cured no mixing
- Two-paste system – 1.5–2.5 minutes: light curing material – immediately upon polymerisation
Figure 7.2 (a) Calcium hydroxide set-up. (b) Step 1 – equal volumes of the base and catalyst are dispensed. (c) Step 2 – final mixed material with a homogenous colour achieved.
Instruments and materials used in set-up
- Calcium hydroxide material
- Waxed paper mixing pad
- Mixing spatula
- Calcium hydroxide applicator
- Gauze
Optional:
- Curing light
- Amber light protection shield
CAVITY VARNISH
Cavity varnish is a liquid resin that is used to seal exposed dentinal tubules to reduce sensitivity due to microleakage, to prevent acids from materials entering tubules and to prevent discolouration of the tooth from the metal ions in amalgam. The solvent constituent of the liquid dissolves once the cavity varnish is applied to the tooth surface, which leaves behind a porous layer. Multiple layers may be applied to reach the desired barrier, drying in between applications. Liquid solvent may be added to the cavity varnish to thin it, if it becomes too thick.
Material constituents/composition
Copal resin in a volatile solvent.
Properties
- The solvent constituent evaporates rapidly, leaving the thin insoluble layer sealing the dentinal tubules
- Physical adhesion to tooth structure
- Does not provide thermal insulation
- Non-irritating and non-acidic
- Strong odour and taste
- To be added in very thin layers, allowing to dry in between
Indications and contraindications for use
Indications
- For use under amalgam and to seal the external surface of newly placed glass ionomer restorations
Contraindications
- Contraindicated with the use of resin composites, polymers and glass ionomers (inhibits bonding)
Trade names
| Trade name | Manufacturer |
| Cavity VarnishTM (Figure 7.3a) | Bosworth |
| Copalite | (Cooley and Cooley) |
Figure 7.3 (a) Cavity varnishTM – Bosworth. (b) Cavity varnish set-up.

Manipulation (Figure 7.3b)
Wearing personal protective equipment:
- There is no mixing necessary with cavity varnish
- Remove the cap of the bottle
- Using sterile college tweezers and a cotton pellet, or disposable applicator brush (preferred), dip the cotton pellet/disposable applicator into the cavity varnish and dab off the excess
- Replace the cap immediately as cavity varnish evaporates quickly
- Pass the college tweezers and cotton pellet/disposable applicator brush to the operator
- The operator will dry the surface in between applications using a 3-in-1 syringe/air-water syringe
- Using separate sterile college tweezers and cotton pellet(s) or new disposable applicator brush(es), repeat step 2 until the desired number of coats has been achieved
- Dispose of the cotton pellet(s) and/or disposable applicator brush(es) in the contaminated waste
Instruments and materials used in set-up
- Mouth mirror and handle
- Cavity varnish material
- 2 X college tweezers (locking type preferable)
- Cotton pellets or disposable applicator brushes
- 3-in-1 syringe/air-water syringe
ZINC PHOSPHATE
Zinc phosphate may be used as an insulating base or a luting cement. Zinc phosphate is a material with a moderate solubility as a base and a high solubility as a luting cement. In this chapter, it will be discussed in the context of a base only.
Constituents
Zinc phosphate is supplied in a powder/liquid form and is available in different shades.
| Powder (base) | Liquid (acid) |
| Zinc oxide | Phosphoric acid |
| Magnesium oxide | Water |
| Aluminium and zinc ions |
Properties
During manipulation of zinc phosphate, once the powder and liquid are mixed together, heat is produced, i.e. an exothermic reaction takes place. This exothermic reaction speeds up the setting of the material. To control the setting of zinc phosphate, it is always mixed on a cool, dry glass slab, and the whole surface area of the slab should be used during the mix to minimize heat production. The manipulation technique is very important as a warm slab, mixing too fast or contamination by water will speed up the setting time of the material. Incorporating the powder increments too fast or too slow will also affect the setting of zinc phosphate. Zinc phosphate is fast setting and has a moderate to high solubility and low acidity (once set). The pH is 1–2 but the acidity decreases over time (about 24 hours).
- Aci/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


