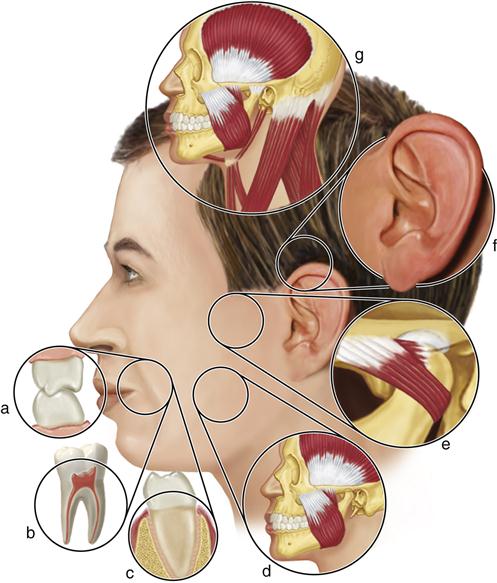
Etiology of Functional Disturbances in the Masticatory System
“THE CLINICIAN WHO ONLY LOOKS AT OCCLUSION IS MISSING AS MUCH AS THE CLINICIAN WHO NEVER LOOKS AT OCCLUSION.”
—JPO
IN THE PRECEDING SIX chapters a detailed description of the optimal anatomy and physiology of occlusion was presented. The discussion ranged from the exact contact and movement of a single tooth to the function of all structures that make up the masticatory system. The optimal functional occlusion was also presented. However, one must question the prevalence of this condition as well as the consequences that arise, if any, when less than ideal conditions exist.
Many symptoms may arise from overloading of the masticatory structures (Figure 7-1). These must be appreciated by the clinician so that the disorders can be identified and managed. However not all signs and symptoms felt in the orofacial structures are related to masticatory dysfunction.
This chapter addresses various functional disturbances in the masticatory system and reviews the specific relationships of the etiologic factors that cause these disturbances.
Terminology
Over the years functional disturbances of the masticatory system have been identified by a variety of terms. This has certainly contributed to some of the confusion in this area. In 1934 James Costen1 described a group of symptoms centering around the ear and temporomandibular joint (TMJ). Because of his work, the term Costen syndrome developed. Later the term TMJ disturbances became popular, and then, in 1959, Shore2 introduced the term TMJ dysfunction syndrome. Later came the term functional TMJ disturbances, coined by Ramfjord and Ash.3 Some terms, such as occlusomandibular disturbance4 and myoarthropathy of the TMJ, described the suggested etiologic factors.5 Others stressed pain, such as pain-dysfunction syndrome,6 myofascial pain-dysfunction syndrome,7 and TM pain-dysfunction syndrome.8
Since the symptoms are not always isolated to the TMJ, some authors believe that the foregoing are too limited and that a broader more collective term should be used, such as craniomandibular disorders.9 Bell10 suggested the term TM disorders, which has gained popularity. This term does not suggest merely problems that are isolated to the TMJs but includes all disturbances associated with the function of the masticatory system.
This wide variety of terms has contributed to the great confusion that exists in this already complicated field of study. Lack of communication and coordination of research efforts often begins with differences in terminology. In an attempt to coordinate efforts, therefore, the American Dental Association11 adopted the term temporomandibular disorders, or TM disorders. In this text, TM disorders is used to include all functional disturbances of the masticatory system.
History of Temporomandibular Disorders
The dental profession generally was first drawn into the area of TM disorders (TMDs) by Costen’s 1934 article.1 Costen was an otolaryngologist who, based on 11 cases, first suggested that changes in dental condition were responsible for various ear symptoms. Not long after Costen’s article appeared, clinicians began to question the accuracy of his conclusions regarding etiology and treatment.12–15 Although most of Costen’s original proposals have been disproved, the dental profession’s interest was certainly stimulated by his work. From the late 1930s and through the 1940s, only a few dentists became interested in managing these pain problems. The most common therapies provided at that time were bite-raising appliances, which were first suggested and described by Costen himself.16,17 From the late 1940s and into the 1950s, the dental profession began to question bite-raising appliances as the therapy of choice for mandibular dysfunction.15,18 It was at this time that the profession began to look more closely at occlusal interferences as the major etiologic factors in complaints suggesting TMD.19,20
Scientific investigation of TMDs first began in the 1950s. Early scientific studies suggested that the occlusal condition could influence masticatory muscle function. Electromyographic studies were used to correlate such relationships.20–22 In the late 1950s, the first textbooks describing masticatory dysfunctions were written.2,8,23 The most common conditions described at that time were masticatory muscle pain disorders. The etiology of these disorders was generally thought to be occlusal disharmony. Occlusion and later emotional stress were accepted as the major etiologic factors of functional disorders of the masticatory system through the 1960s and into the 1970s. Then, in the late 1970s, there was an explosion of interest in TMDs. At this time also, information reached the profession concerning pain disorders arising from intracapsular sources.24 This information reoriented the profession’s thinking and direction in the area of TMDs. It was not until the 1980s that the profession began to recognize fully and appreciate the complexity of TMDs. This complexity now has the profession striving to find its proper role in the management of TMDs and orofacial pains.25
Early in the development of the field of TMD and orofacial pain there was little research, nor was there an appreciation of evidence-based medicine. Consequently treatments were instituted that were often not effective and sometimes were very aggressive. During the 1990s and 2000s the profession came to embrace the concept of evidence-based medicine, and with this came the need for training programs to better prepare clinicians for managing their TMD patients. Postgraduate training programs were begun in several universities formalizing this educational process. In 2010 the Commission on Dental Accreditation, the agency charged with accrediting all dental specialties in the United States, acknowledged the need to recognize and standardize these programs. At that time these university-based programs began an accreditation process similar to those for other dental specialties. It is hoped that these advancements in research and education will greatly improve the diagnosis and management of TMD, providing improved quality of life for the many individuals suffering with TMD and orofacial pain.
Epidemiologic Studies of Temporomandibular Disorders
For the study of TMDs to have a place in the practice of dentistry, TMD must first be shown to represent a significant problem in the general population; second, it must relate to structures treated by the dentist. If signs and symptoms of masticatory dysfunction are common in the general population, TMD becomes an important problem that must be addressed. Studies that examine these signs and symptoms are discussed below.
If TMD symptoms do prove to be common, then one must next ask “What is the etiology of TMD and can it be treated by dental therapies?” The question of etiology must be discussed here since it is basic to an understanding of the dentist’s role in managing TMD. The question of therapy is addressed in later chapters. Many dentists feel that the occlusion of the teeth is the primary etiology of TMD symptoms. This question has been highly debated in dentistry since the days of Costen. If occlusion does play a significant role in the etiology of TMD, the dentist can and should play an important role in the management of these disorders. No other health care providers can provide this treatment. On the other hand, if occlusion plays no role in TMD, any attempt by the dentist to alter the occlusal condition is misdirected and should be avoided. It becomes obvious that this question is very important to the dental profession. One of the goals of this chapter is to explore the scientific studies that offer insight into this most important question.
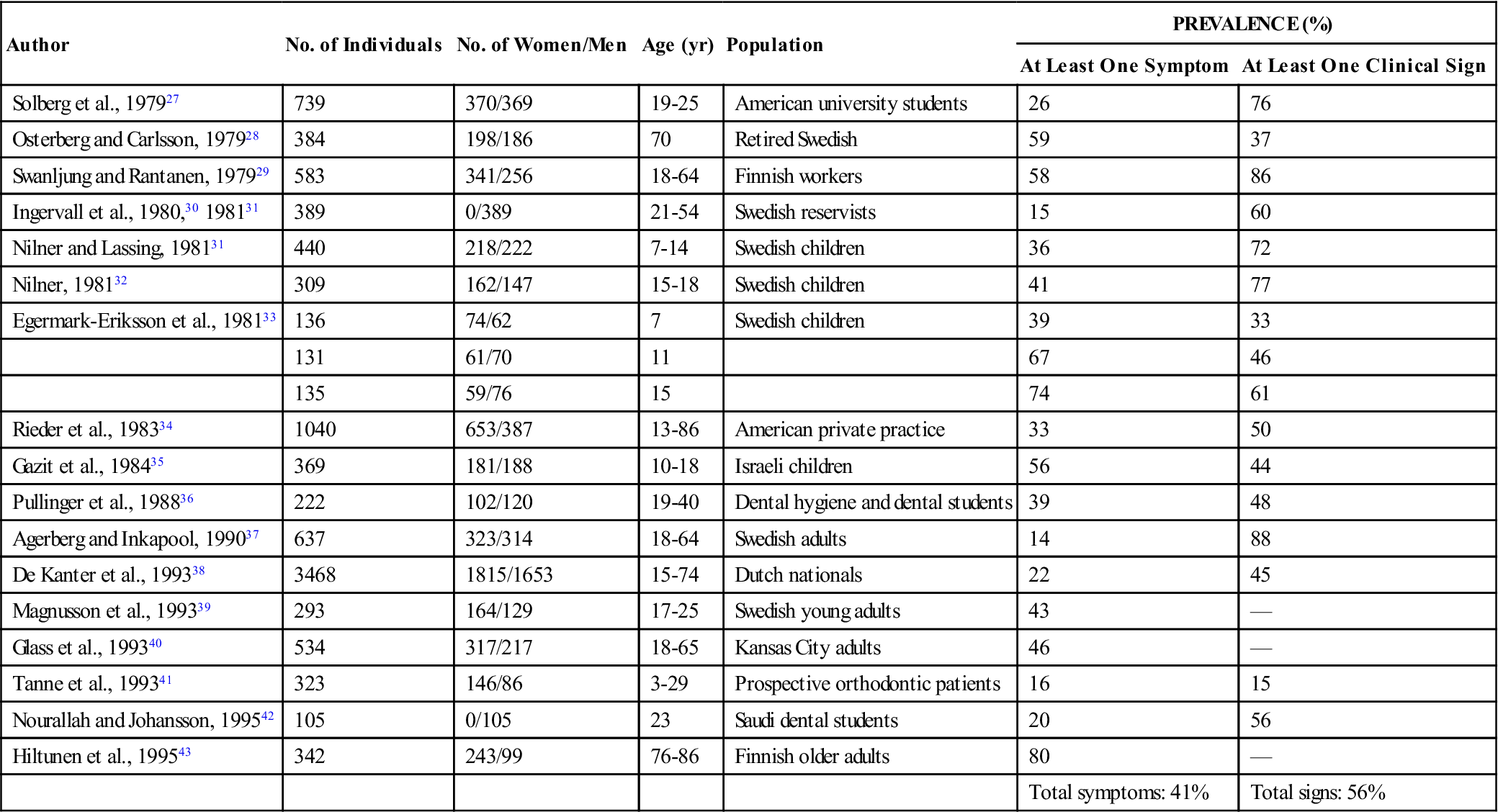
The prevalence of signs and symptoms associated with TMD can best be appreciated by examining epidemiologic studies. Dorland’s Medical Dictionary describes epidemiology as “the science concerned with the study of the factors determining and influencing the frequency and distribution of disease, injury, and other health-related events and their causes in a defined human population for the purpose of establishing programs to prevent and control their development and spread.”26 Numerous epidemiologic studies have examined the prevalence of TMDs in given populations. A few of these are summarized in Table 7-1.27–43 In each study subjects were questioned regarding symptoms and then examined for common clinical signs associated with TMD. The results are found under “Prevalence” in the right-hand column of Table 7-1. The number there represents the percentage of subjects who had at least one clinical symptom or one clinical sign relating to TMD. These studies certainly suggest that signs and symptoms of TMDs are quite common in these populations. In fact, an average of 41% of these populations reported at least one symptom associated with TMD, while an average of 56% showed at least one clinical sign. Since these studies ranged through many age and sex distributions, it is probably safe to assume that a similar percentage also exists in the general population. According to these studies, it would seem that a conservative estimate of the percentage of people in the general population with some type of TMD ranges between 40% and 60%. This figure is so high that it might lead one to doubt the validity of the studies. After all, half the patients seen in a dental office do not appear to be suffering from TMD.
TABLE 7-1
Signs and Symptoms of Temporomandibular Disorders in Investigated Populations
| Author | No. of Individuals | No. of Women/Men | Age (yr) | Population | PREVALENCE (%) | |
| At Least One Symptom | At Least One Clinical Sign | |||||
| Solberg et al., 197927 | 739 | 370/369 | 19-25 | American university students | 26 | 76 |
| Osterberg and Carlsson, 197928 | 384 | 198/186 | 70 | Retired Swedish | 59 | 37 |
| Swanljung and Rantanen, 197929 | 583 | 341/256 | 18-64 | Finnish workers | 58 | 86 |
| Ingervall et al., 1980,30 198131 | 389 | 0/389 | 21-54 | Swedish reservists | 15 | 60 |
| Nilner and Lassing, 198131 | 440 | 218/222 | 7-14 | Swedish children | 36 | 72 |
| Nilner, 198132 | 309 | 162/147 | 15-18 | Swedish children | 41 | 77 |
| Egermark-Eriksson et al., 198133 | 136 | 74/62 | 7 | Swedish children | 39 | 33 |
| 131 | 61/70 | 11 | 67 | 46 | ||
| 135 | 59/76 | 15 | 74 | 61 | ||
| Rieder et al., 198334 | 1040 | 653/387 | 13-86 | American private practice | 33 | 50 |
| Gazit et al., 198435 | 369 | 181/188 | 10-18 | Israeli children | 56 | 44 |
| Pullinger et al., 198836 | 222 | 102/120 | 19-40 | Dental hygiene and dental students | 39 | 48 |
| Agerberg and Inkapool, 199037 | 637 | 323/314 | 18-64 | Swedish adults | 14 | 88 |
| De Kanter et al., 199338 | 3468 | 1815/1653 | 15-74 | Dutch nationals | 22 | 45 |
| Magnusson et al., 199339 | 293 | 164/129 | 17-25 | Swedish young adults | 43 | — |
| Glass et al., 199340 | 534 | 317/217 | 18-65 | Kansas City adults | 46 | — |
| Tanne et al., 199341 | 323 | 146/86 | 3-29 | Prospective orthodontic patients | 16 | 15 |
| Nourallah and Johansson, 199542 | 105 | 0/105 | 23 | Saudi dental students | 20 | 56 |
| Hiltunen et al., 199543 | 342 | 243/99 | 76-86 | Finnish older adults | 80 | — |
| Total symptoms: 41% | Total signs: 56% | |||||
To appreciate these percentages better, one must examine the studies more closely. The study by Solberg et al27 can be helpful in appreciating the prevalence of TMD. In this study the investigators examined 739 university students (aged 18-25) who were reporting to a student health clinic for enrollment in a health insurance program. A questionnaire was completed and a short clinical examination performed to identify any signs or symptoms related to TMD. A sign was considered to be any clinical finding associated with a TMD. A symptom was any sign of which the patient was aware and which was therefore reported. The clinical examination revealed that 76% of the students had one or more signs associated with TMDs. The questionnaire, however, revealed that only 26% of the students reported having a symptom that related to TMD. In other words, 50% of the group had signs that were not reported as symptoms. Signs that are present but unknown to the patient are called subclinical. It was also reported that only 10% of the total group had symptoms that were severe enough to cause the patient to seek treatment. Only 5% made up a group that would be typically described as TMD patients with severe problems such as would be seen in dental offices. These kinds of findings are more readily accepted as factual. In other words, one out of every four patients in a general population will report some awareness of TMD symptoms, yet less than 10% of the population studied felt that their problem was severe enough to call for treatment.44–49 The major factor that seemed to determine whether professional care would be sought was the degree of pain being experienced.50 It must not be forgotten, however, that all these studies report an average of 40% to 60% of the population as having at least one detectable sign associated with TMD. Other studies have confirmed these findings.51–59
Although children and young adults do reveal an increase in signs of TMD as they age, they rarely complain of any significant symptoms.60 In a similar finding, patients who are 60 years of age or older also rarely complain of TMD symptoms.61–64 Epidemiologic studies reveal that the most TMD symptoms are reported by people in the 20- to 40-year age group.62,65–66 The possible reasons for this are discussed in later chapters.
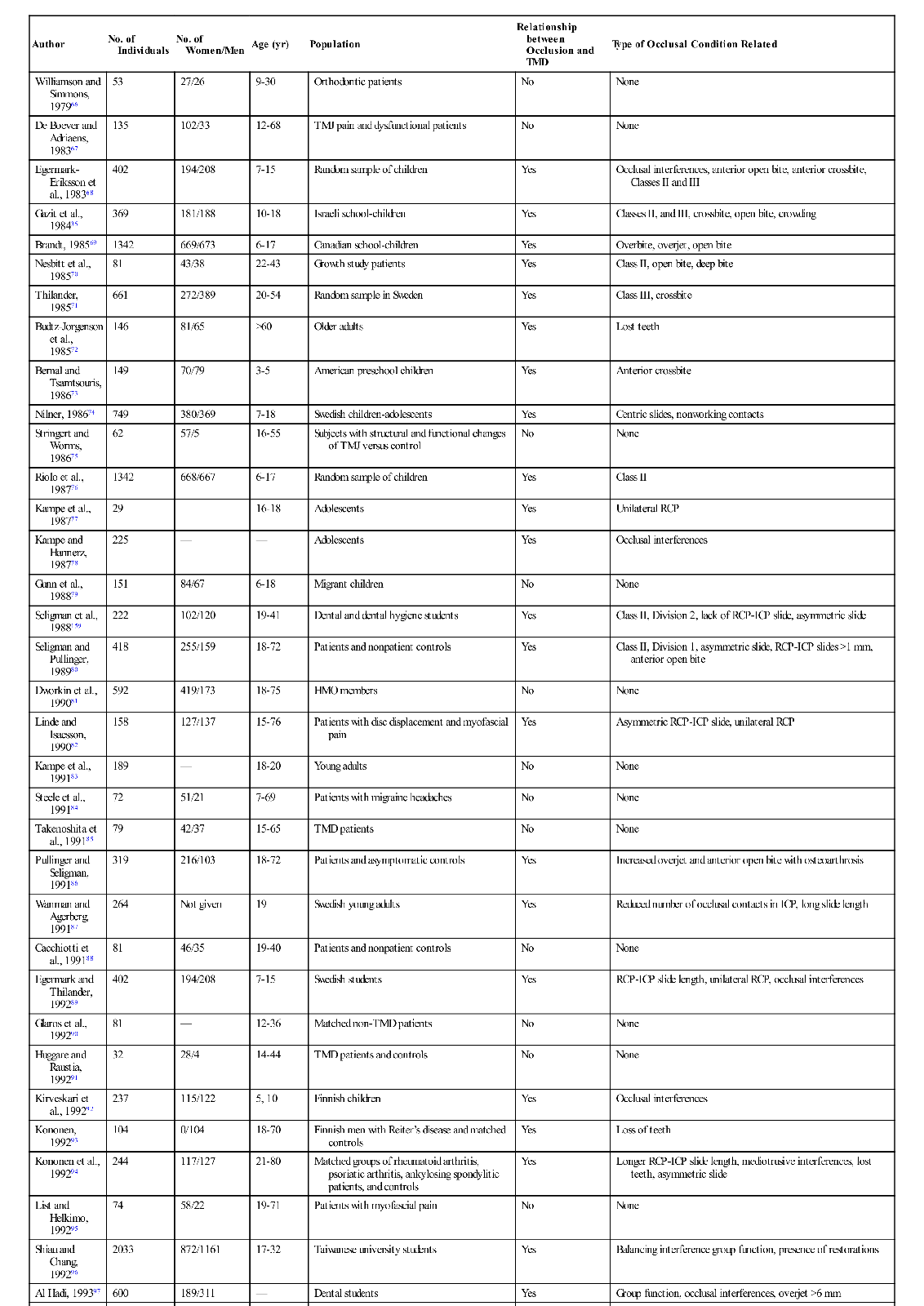
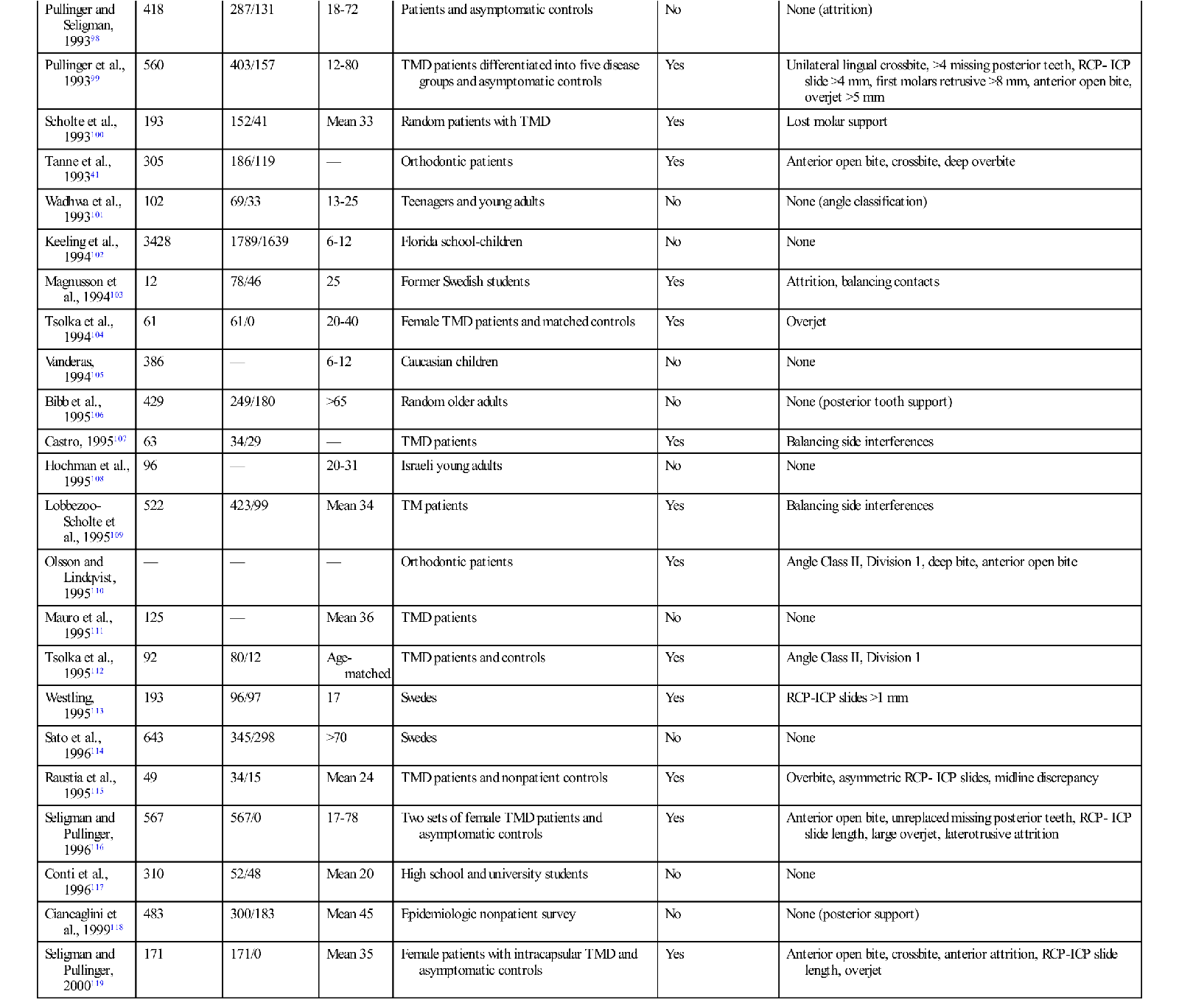
The studies outlined above reveal that the prevalence of functional disorders in the masticatory system is high, especially in certain populations. Since it is documented that occlusal contact patterns influence the function of the masticatory system (Chapter 2), a logical assumption would be that the occlusal contact pattern may also influence functional disturbances. If this relationship is correct, it makes the study of occlusion a significant and important part of dentistry. The relationship between occlusion and TMD, however, is not simple. Table 7-2 summarizes 57 epidemiologic studies of a variety of populations that attempted to look at the relationship between occlusion and the signs and symptoms associated with TMD.35,36,41,67–121 In this table, any significant relationship found between occlusal factors and TMD are described in the right column. Where no relationship was found, the word no appears in the column. It should be noted that 22 of these studies found no relationship between occlusal factors and TMD symptoms, whereas 35 studies did find such a relationship. The fact that these studies do not consistently report a common relationship explains why the subject of occlusion and TMD has been the focus of so much controversy and debate. In fact, if occlusal factors were either the main cause of TMD or if occlusion had nothing to do with TMD, we should probably expect to see more agreement in the findings. One might conclude that if occlusion were the major etiologic factor in TMD, the profession would have confirmed this many years ago. On the other hand, if occlusion has nothing to do with TMD, the profession would have also likewise already confirmed this conclusion. Apparently neither of these conclusions is true. Instead, the confusion and controversy concerning the relationship between occlusion and TMD continues. The general message is that there is no simple cause-and-effect relationship explaining the association between occlusion and TMD.
TABLE 7-2
| Author | No. of Individuals | No. of Women/Men | Age (yr) | Population | Relationship between Occlusion and TMD | Type of Occlusal Condition Related |
| Williamson and Simmons, 197966 | 53 | 27/26 | 9-30 | Orthodontic patients | No | None |
| De Boever and Adriaens, 198367 | 135 | 102/33 | 12-68 | TMJ pain and dysfunctional patients | No | None |
| Egermark-Eriksson et al., 198368 | 402 | 194/208 | 7-15 | Random sample of children | Yes | Occlusal interferences, anterior open bite, anterior crossbite, Classes II and III |
| Gazit et al., 198435 | 369 | 181/188 | 10-18 | Israeli school-children | Yes | Classes II, and III, crossbite, open bite, crowding |
| Brandt, 198569 | 1342 | 669/673 | 6-17 | Canadian school-children | Yes | Overbite, overjet, open bite |
| Nesbitt et al., 198570 | 81 | 43/38 | 22-43 | Growth study patients | Yes | Class II, open bite, deep bite |
| Thilander, 198571 | 661 | 272/389 | 20-54 | Random sample in Sweden | Yes | Class III, crossbite |
| Budtz-Jorgenson et al., 198572 | 146 | 81/65 | >60 | Older adults | Yes | Lost teeth |
| Bernal and Tsamtsouris, 198673 | 149 | 70/79 | 3-5 | American preschool children | Yes | Anterior crossbite |
| Nilner, 198674 | 749 | 380/369 | 7-18 | Swedish children-adolescents | Yes | Centric slides, nonworking contacts |
| Stringert and Worms, 198675 | 62 | 57/5 | 16-55 | Subjects with structural and functional changes of TMJ versus control | No | None |
| Riolo et al., 198776 | 1342 | 668/667 | 6-17 | Random sample of children | Yes | Class II |
| Kampe et al., 198777 | 29 | — | 16-18 | Adolescents | Yes | Unilateral RCP |
| Kampe and Hannerz, 198778 | 225 | — | — | Adolescents | Yes | Occlusal interferences |
| Gunn et al., 198879 | 151 | 84/67 | 6-18 | Migrant children | No | None |
| Seligman et al., 1988159 | 222 | 102/120 | 19-41 | Dental and dental hygiene students | Yes | Class II, Division 2, lack of RCP-ICP slide, asymmetric slide |
| Seligman and Pullinger, 198980 | 418 | 255/159 | 18-72 | Patients and nonpatient controls | Yes | Class II, Division 1, asymmetric slide, RCP-ICP slides >1 mm, anterior open bite |
| Dworkin et al., 199081 | 592 | 419/173 | 18-75 | HMO members | No | None |
| Linde and Isacsson, 199082 | 158 | 127/137 | 15-76 | Patients with disc displacement and myofascial pain | Yes | Asymmetric RCP-ICP slide, unilateral RCP |
| Kampe et al., 199183 | 189 | — | 18-20 | Young adults | No | None |
| Steele et al., 199184 | 72 | 51/21 | 7-69 | Patients with migraine headaches | No | None |
| Takenoshita et al., 199185 | 79 | 42/37 | 15-65 | TMD patients | No | None |
| Pullinger and Seligman, 199186 | 319 | 216/103 | 18-72 | Patients and asymptomatic controls | Yes | Increased overjet and anterior open bite with osteoarthrosis |
| Wanman and Agerberg, 199187 | 264 | Not given | 19 | Swedish young adults | Yes | Reduced number of occlusal contacts in ICP, long slide length |
| Cacchiotti et al., 199188 | 81 | 46/35 | 19-40 | Patients and nonpatient controls | No | None |
| Egermark and Thilander, 199289 | 402 | 194/208 | 7-15 | Swedish students | Yes | RCP-ICP slide length, unilateral RCP, occlusal interferences |
| Glaros et al., 199290 | 81 | — | 12-36 | Matched non-TMD patients | No | None |
| Huggare and Raustia, 199291 | 32 | 28/4 | 14-44 | TMD patients and controls | No | None |
| Kirveskari et al., 199292 | 237 | 115/122 | 5, 10 | Finnish children | Yes | Occlusal interferences |
| Kononen, 199293 | 104 | 0/104 | 18-70 | Finnish men with Reiter’s disease and matched controls | Yes | Loss of teeth |
| Kononen et al., 199294 | 244 | 117/127 | 21-80 | Matched groups of rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitic patients, and controls | Yes | Longer RCP-ICP slide length, mediotrusive interferences, lost teeth, asymmetric slide |
| List and Helkimo, 199295 | 74 | 58/22 | 19-71 | Patients with myofascial pain | No | None |
| Shiau and Chang, 199296 | 2033 | 872/1161 | 17-32 | Taiwanese university students | Yes | Balancing interference group function, presence of restorations |
| Al Hadi, 199397 | 600 | 189/311 | — | Dental students | Yes | Group function, occlusal interferences, overjet >6 mm |
| Pullinger and Seligman, 199398 | 418 | 287/131 | 18-72 | Patients and asymptomatic controls | No | None (attrition) |
| Pullinger et al., 199399 | 560 | 403/157 | 12-80 | TMD patients differentiated into five disease groups and asymptomatic controls | Yes | Unilateral lingual crossbite, >4 missing posterior teeth, RCP- ICP slide >4 mm, first molars retrusive >8 mm, anterior open bite, overjet >5 mm |
| Scholte et al., 1993100 | 193 | 152/41 | Mean 33 | Random patients with TMD | Yes | Lost molar support |
| Tanne et al., 199341 | 305 | 186/119 | — | Orthodontic patients | Yes | Anterior open bite, crossbite, deep overbite |
| Wadhwa et al., 1993101 | 102 | 69/33 | 13-25 | Teenagers and young adults | No | None (angle classification) |
| Keeling et al., 1994102 | 3428 | 1789/1639 | 6-12 | Florida school-children | No | None |
| Magnusson et al., 1994103 | 12 | 78/46 | 25 | Former Swedish students | Yes | Attrition, balancing contacts |
| Tsolka et al., 1994104 | 61 | 61/0 | 20-40 | Female TMD patients and matched controls | Yes | Overjet |
| Vanderas, 1994105 | 386 | — | 6-12 | Caucasian children | No | None |
| Bibb et al., 1995106 | 429 | 249/180 | >65 | Random older adults | No | None (posterior tooth support) |
| Castro, 1995107 | 63 | 34/29 | — | TMD patients | Yes | Balancing side interferences |
| Hochman et al., 1995108 | 96 | — | 20-31 | Israeli young adults | No | None |
| Lobbezoo-Scholte et al., 1995109 | 522 | 423/99 | Mean 34 | TM patients | Yes | Balancing side interferences |
| Olsson and Lindqvist, 1995110 | — | — | — | Orthodontic patients | Yes | Angle Class II, Division 1, deep bite, anterior open bite |
| Mauro et al., 1995111 | 125 | — | Mean 36 | TMD patients | No | None |
| Tsolka et al., 1995112 | 92 | 80/12 | Age- matched | TMD patients and controls | Yes | Angle Class II, Division 1 |
| Westling, 1995113 | 193 | 96/97 | 17 | Swedes | Yes | RCP-ICP slides >1 mm |
| Sato et al., 1996114 | 643 | 345/298 | >70 | Swedes | No | None |
| Raustia et al., 1995115 | 49 | 34/15 | Mean 24 | TMD patients and nonpatient controls | Yes | Overbite, asymmetric RCP- ICP slides, midline discrepancy |
| Seligman and Pullinger, 1996116 | 567 | 567/0 | 17-78 | Two sets of female TMD patients and asymptomatic controls | Yes | Anterior open bite, unreplaced missing posterior teeth, RCP- ICP slide length, large overjet, laterotrusive attrition |
| Conti et al., 1996117 | 310 | 52/48 | Mean 20 | High school and university students | No | None |
| Ciancaglini et al., 1999118 | 483 | 300/183 | Mean 45 | Epidemiologic nonpatient survey | No | None (posterior support) |
| Seligman and Pullinger, 2000119 | 171 | 171/0 | Mean 35 | Female patients with intracapsular TMD and asymptomatic controls | Yes | Anterior open bite, crossbite, anterior attrition, RCP-ICP slide length, overjet |
Contributions to this table from Dr. James McNamara, University of Michigan, Ann Arbor; and Dr. Donald Seligman, Los Angeles.120
HMO, Health maintenance organization; ICP, intercuspal position; RCP, retruded contact position; TMD, temporomandibular disorder; TMJ, temporomandibular joint.
However, another 35 studies did find a relationship between occlusion and TMD, leading to the question “What was the significant occlusal relationship that was found to be related to TMD symptoms?” As one can see in Table 7-2, no consistent occlusal conditions were reported in these studies. In fact there are a variety of conditions, the incidence of which varies greatly from study to study. These findings make it even more difficult to understand the relationship between occlusion and TMD.
Most clinicians would also agree that the occlusal conditions found in these studies do not always lead to TMD symptoms. In fact, these findings are commonly seen in symptom-free populations. To appreciate the role of occlusion in TMD, one must better understand the many factors that can influence function of this extremely complex system.
Etiologic Considerations in Temporomandibular Disorders
Although signs and symptoms of disturbances in the masticatory system are common, understanding etiology can be very complex. There is no single etiology that accounts for all signs and symptoms. For example, if one refers to a medical textbook for suggested treatments of a disorder and only one therapy is listed, one usually will find that this treatment is quite effective. On the other hand, if the textbook lists multiple treatments for the same disorder, the therapist can assume that none of the suggested therapies will always be effective. There are two explanations for these findings. Either the disorder has multiple etiologies and no single treatment can affect all the etiologies, or the disorder is not a single problem but represents an umbrella term under which there are multiple disorders. In the case of TMD, both these explanations are true. Certainly a multitude of conditions can affect masticatory function. Also, according to the structures involved, a variety of disorders can result.
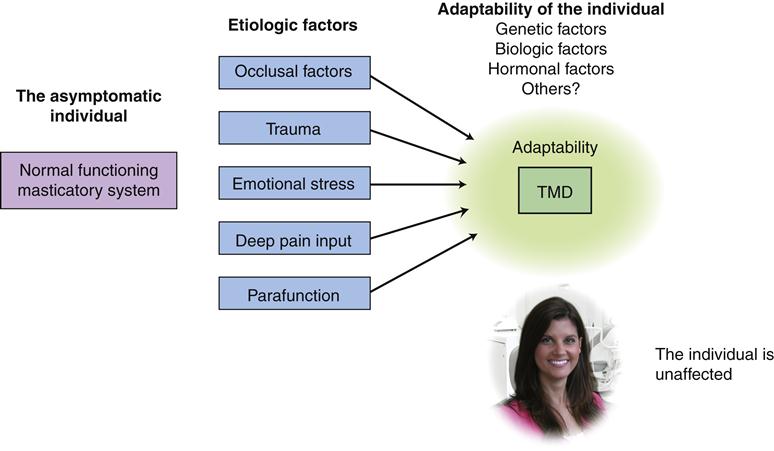
The etiology of TMDs is complex and multifactorial. Numerous factors can contribute to TMD. Factors that increase the risk of TMD are called predisposing factors. Factors that cause the onset of TMD are called initiating factors, and factors that interfere with healing or enhance the progression of TMD are called perpetuating factors. In some instances, a single factor may serve one or all of these roles.9,122,123 The successful management of TMD is dependent upon identifying and controlling these contributing factors.
The dentist attempting to manage a TMD patient must appreciate the major etiologic factors that may be associated with the condition. This is essential for selecting proper and effective therapy. To assist in this understanding, Figure 7-2 depicts the interrelationships between all the associated factors.
Normal Function
As discussed in Chapter 2, the masticatory system is a complex unit designed to carry out the tasks of chewing, swallowing, and speaking. These functions are basic to life. These tasks are carried out by the complex neuromuscular control system. As previously discussed, the brainstem (specifically the central pattern generator) regulates muscle action by way of muscle engrams that are appropriately selected according to sensory input received from the peripheral structures. When sudden, unexpected sensory input is received, protective reflex mechanisms are activated, creating a decrease in muscle activity in the area of the input. A more complete review of this nociceptive reflex and its normal function was provided in Chapter 2.
In most individuals the masticatory system functions normally and efficiently without significant consequence. However, throughout life, certain factors can interrupt normal function, creating dysfunction of the masticatory structures. These factors are called etiologic factors.
Etiologic Factors Underlying Temporomandibular Disorders
It is appropriate to begin with a thorough discussion of the major etiologic factors leading to TMD. Proper identification of the correct factors is basic for therapeutic success. A review of the scientific literature reveals five major factors associated with TMD. These factors are the occlusal condition, trauma, emotional stress, deep pain input, and parafunctional activities. The importance of any of these factors may vary greatly from patient to patient. Each factor is discussed here. However, because occlusal factors are so important in dentistry, their precise effect on TMD is detailed later in this chapter.
The occlusal condition as an etiology of temporomandibular disorder
One contributing factor to TMD that has been strongly debated for many years is the occlusal condition. Early in the development of this field the profession was very convinced that occlusion was the most important contributing factor in TMD. More recently many researchers have argued that occlusal factors play little to no role in TMD. Certainly the research data reviewed above do not present overwhelming evidence for either side of this debate. The relationship of occlusal factors in TMD, however, is an extremely critical issue in dentistry. If occlusal factors are related to TMD, the dentist is responsible for providing proper therapy, since dentists are the only health care professionals trained to change the occlusion. On the other hand, if occlusal factors are not related to TMD, the dentist should refrain from treating TMD with occlusal changes. One can understand the importance of this issue and therefore how highly emotional the debate has become. This relationship is discussed in detail later in this section once a complete graphic depiction has been given.
Trauma as an etiology of temporomandibular disorder
Certainly trauma to the facial structures can lead to functional disturbances in the masticatory system. There is ample evidence supporting this concept.124–139 Trauma seems to have a greater impact on intracapsular disorders than on muscular disorders. Trauma can be divided into two general types: macrotrauma and microtrauma. Macrotrauma is any sudden force that can result in structural alterations, such as a direct blow to the face. Microtrauma is any small force that is repeatedly applied to the structures over a long period of time. Activities such as bruxism or clenching can produce microtrauma to the tissues that are being loaded (i.e., teeth, joints, or muscles).140 The specific types and effects of trauma are discussed in Chapter 8.
Emotional stress as an etiology of temporomandibular disorder
A common factor that can influence masticatory function is an increase in the level of emotional stress. As described in Chapter 2, the emotional centers of the brain have an influence on muscle function. The hypothalamus, the reticular system, and particularly the limbic system are primarily responsible for an individual’s emotional state. These centers influence muscle activity in many ways, one of which is through the gamma efferent pathways. Stress affects the body by activating the hypothalamic-pituitary-adrenal (HPA) axis, which in turn prepares the body to respond (through the autonomic nervous system). The HPA axis, through complex neural pathways, increases the activity of the gamma efferents, which cause the intrafusal fibers of the muscle spindles to contract. This so sensitizes the spindles that any slight stretching of the muscle will cause a reflex contraction. The overall effect is an increase in the muscle’s tonicity.141
Emotional stress must be understood and appreciated by the clinician, since it commonly plays an important role in TMD. The patient’s emotional state is largely dependent on the psychological stress being experienced. Stress is described by Hans Selye142 as “the nonspecific response of the body to any demand made upon it.” Psychological stress is an intricate part of our lives. It is not an unusual emotional disturbance isolated to institutionalized patients. Stress is a force that each of us may experience. Contrary to what we might think, it is not always bad. It is often a motivational force driving us to accomplish a task and achieve success. Circumstances or experiences that create stress are known as stressors. These can be unpleasant (like losing one’s job) or pleasant (like leaving for a vacation). As far as the body is concerned, whether the stressor is pleasant or unpleasant is not important.142 The significant fact is that the body reacts to the stressor by creating certain demands for readjustment or adaptation (the “fight-or-flight” response). These demands are related in degree to the intensity of the stressor.
A simple way of describing stress is to consider it as a type of energy. When a stressful situation is encountered, energy is generated within the body and must be released in some way. There are basically two types of releasing mechanisms. The first is external and is represented by activities such as shouting, cursing, hitting, or throwing objects. Although these activities are common and almost a natural response to stress, they are not generally well accepted in our society. External stress-releasing mechanisms are quite natural, as revealed by a young child having a temper tantrum. Since society has classified these as undesirable, we must learn other stress-releasing mechanisms. Another source of external stress release is physical exercise. It would appear that this type of release is a healthy way in which to deal with stress; it is discussed in later chapters.
A second mechanism by which stress is released is an internal mechanism, in which the person releases the stress internally and develops a psychophysiologic disorder such as irritable bowel syndrome, hypertension, certain cardiac arrhythmias, asthma, or an increase in the tonicity of the head and neck musculature. As accurate documentation regarding the prevalence of increased muscle tension is accumulated, it may be learned that this type of stress-releasing mechanism is by far the most common. It is important to remember that the perception of the stressor, in both type and intensity, varies greatly from person to person. What may be stressful for one person quite possibly represents no stress for another. It is difficult, therefore, to judge the intensity of a given stressor on a given patient.
Increased levels of emotional stress experienced not only increases the tonicity of the head and neck muscles141 but it can also increase levels of nonfunctional muscle activity, such as bruxism or tooth clenching.
Emotional stress can also influence the individual’s sympathetic activity or tone. The autonomic nervous system constantly monitors and regulates numerous subconscious systems that maintain homeostasis. One of the functions of the autonomic nervous system is to regulate blood flow within the body. The sympathetic nervous system is closely related to the fight-or-flight reflex activated by stressors. Therefore in the presence of stress the capillary blood flow in the outer tissues is constricted, permitting increased blood flow to the more important musculoskeletal structures and internal organs. The results are a cooling of the skin such as the hands. Prolonged activity of the sympathetic nervous system can affect certain tissues such as the muscles. It has been suggested that sympathetic activity can increase muscle tone,143,144 thereby producing a painful muscle condition. Increased sympathetic activity or tone therefore represents an etiologic factor that can influence TMD symptoms.
As previously mentioned, emotional stress is part of the human existence. We are built to handle stress, as demonstrated by the body’s fight-or-flight response to the challenges of our environment. The acute response to a sudden environmental challenge is healthy and necessary for survival—for example, running from a burning building or jumping away from an oncoming car. The issues of concern are not these acute responses but those that expose us to prolonged emotional stress, especially without the ability to escape—for example, negative work environments, unhappy marriages, or compromised family situations. Prolonged exposure to emotional stressors upregulates the autonomic nervous system on a chronic basis, which can compromise the individual’s ability to adapt and even to fight diseases. It is this link that ties TMD to emotional stress; it is elaborated on in later chapters.
Deep pain input as an etiology of temporomandibular disorder
A common yet often overlooked concept is that sources of deep pain input can cause altered muscle function. This idea is discussed in detail in Chapter 2. Deep pain input can centrally excite the brainstem, producing a muscle response known as protective co-contraction.145 This represents a normal, healthy manner in which the body responds to injury or threat of injury. Therefore it is reasonable to find a patient who is suffering with pain, such as toothache (i.e., necrotic pulp), to have limited mouth opening. This represents the body’s response to protect the injured part by limiting its use. This clinical finding is common in many toothache patients. Once the tooth pain has been resolved, normal mouth opening returns. The limited mouth opening is merely a secondary response to the experience of the deep pain. If the clinician does not recognize this phenomenon, however, he or she may conclude that the limited mouth opening is a primary TMD problem and treatment would be misdirected. Any source of constant deep pain input can represent an etiologic factor that may lead to limited mouth opening and therefore clinically present as TMD. Tooth pain, sinus pain, and ear pain can create this response. Even pain sources remote to the face, such as cervical pain, can lead to this condition (Chapter 2). Too often dentists do not appreciate this phenomenon and begin treating the patient for TMD complaints. Only after treatment failure is the cervical pain condition identified as being responsible for the facial pain and limited mouth opening. An understanding of how this occurs is basic to treatment and emphasizes the importance of making the correct diagnosis (see Chapters 9 and 10).
Parafunctional activity as an etiology of temporomandibular disorder
Activities of the masticatory muscles can be divided into two basic types: functional (described in Chapter 2), including chewing, speaking, and swallowing, and parafunctional (i.e., nonfunctional), including clenching or grinding the teeth (referred to as bruxism) as well as various other oral habits. The term muscle hyperactivity has also been used to describe any increased muscular activity over and above that necessary for function. Muscle hyperactivity thus includes not only the parafunctional activities of clenching, bruxing, and other oral habits but also any general increase in the level of muscle tonus. Some muscle hyperactivity may not even involve tooth contact or jaw movement but may merely represent an increase in the static tonic contraction of the muscle.
Some of these activities may be responsible for creating TMD symptoms.120–146 For purposes of discussion, parafunctional activity can be subdivided into two general types: that which occurs through the day (diurnal) and that which occurs at night (nocturnal).
Diurnal activity
Parafunctional activity during the day consists of clenching and grinding as well as many other oral habits that are often performed without the individual’s awareness, such as cheek and tongue biting, finger and thumb sucking, unusual postural habits, and many occupation-related activities such as biting on pencils, pins, or nails or holding objects under the chin (a telephone or violin). It is common, during daily activities, for individuals to place their teeth together and apply force.147–149 This type of diurnal activity may be seen in someone who is concentrating on a task or performing a strenuous physical chore. The masseter muscle contracts periodically in a manner that is totally irrelevant to the task at hand. Such irrelevant activity, already described in Chapter 2, is commonly associated with many daytime tasks (e.g., driving a car, reading, writing, typing, lifting a heavy object). Some diurnal activities are very closely related to the task being accomplished, such as when an underwater diver bites on his or her mouthpiece150 or musicians play certain musical instruments.151,152
The clinician must recognize that most parafunctional activities occur at a subconscious level. In other words, individuals are often not aware of their clenching or cheek-biting habits (Figure 7-3). Therefore merely questioning the patient is not a reliable way to assess the presence or absence of these activities.153 In many instances, once the clinician makes the patient aware of the possibility of these diurnal activities, he or she will recognize them and can than decrease them. This is the best treatment strategy that can be provided and is discussed further in later chapters.
Nocturnal activity
Data from various sources suggest that parafunctional activity during sleep is quite common154–157 and seems to take the form of single episodes (referred to as clenching) and rhythmic contractions (known as bruxing). Whether these activities result from different etiologic factors or are the same phenomenon in two different presentations is not known. In many patients both activities occur and are sometimes difficult to separate. For that reason clenching and bruxism are often referred as bruxing events.
Sleep
To best understand nocturnal bruxism, one should first have an appreciation of the sleep process. Sleep is investigated by monitoring the electroencephalographic (EEG) brain wave activity of an individual during sleep. This monitoring is called polysomnography. A polysomnogram reveals two basic types of brainwave activities that appear to cycle during a night of sleep. The first type is a relatively fast wave called an alpha wave (about 10 waves per second). Alpha waves are the predominant waves observed during the early stages of sleep or light sleep. Delta waves are slower waves (0.5-4 waves per second) observed during the deeper stages of sleep. The sleep cycle is divided into four stages of non–rapid-eye-movement (non-REM) sleep followed by a period of REM sleep. Stages 1 and 2 represent the early phases of light sleep and are made up of groups of fast alpha waves along with a few beta waves and sleep spindles. Stages 3 and 4 represent the deeper stages of sleep with a predominance of the slower beta waves.
During a normal cycle of sleep, a subject will pass from the light stages, 1 and 2, into the deeper stages 3, and 4. The subject will then pass through a stage of sleep that is quite different from the others. This stage appears as a desynchronized activity in which other physiologic events occur, such as twitching of the muscles of the face and extremities, alterations in the heart rhythm and rate of breathing, and rapid movement of the eyes beneath the eyelids158—thus the name REM sleep. It is during REM sleep that dreaming most commonly occurs. After the REM period the person typically moves back into a lighter stage, and the cycle repeats itself throughout the night. Each complete cycle of sleep takes from 60 to 90 minutes, resulting in an average of between four and six cycles of sleep per night. An REM phase usually occurs following stage 4 sleep and lasts from 5 to 15 minutes. Some 80% of people who are awakened during REM sleep can recall the dreams they were experiencing.159 Only 5% of those awakened during non-REM phases can recall their dreams (some can recall them partially).
Approximately 80% of the sleep period of an adult is made up of non-REM sleep, with only 20% being REM sleep.160 Because REM and non-REM sleep appear to be so different, it is thought that their functions are also quite different. Non-REM sleep is thought to be important in restoring the function of body systems. During this phase of sleep there is an increase in synthesis of vital macromolecules (i.e., proteins, RNA, etc.). REM sleep, on the other hand, seems to be important in restoring the function of the cerebral cortex and brainstem. It is thought that during this phase of sleep emotions are dealt with and smoothed out. At this time the meaning of recent experiences are brought into alignment with old pathways.
The importance of these two types of sleep is evident from studies that attempt to deprive individuals of one or the other. When an individual is experimentally deprived of REM sleep, certain emotional states become predominant.161 The subjects show greater anxiety and irritability. They also have difficulty concentrating. It would appear that REM sleep is important for psychic rest. A different finding is revealed when an individual is deprived of non-REM sleep.162–164 When a normal subject is experimentally deprived of non-REM sleep for several nights, he or she will often begin to complain of musculoskeletal tenderness, aching, and stiffness.165 This may result from the individual’s inability to restore metabolic requirements. In other words, non-REM sleep is important for physical rest.
In one study experimental deprivation of non-REM sleep did not seem to increase the EMG activity of the elevator muscles during sleep.166 Therefore there is still debate as to why deprivation of non-REM sleep leads to musculoskeletal tenderness, aching, and stiffness.167 Nonetheless, it is very important that the clinician treating TMDs have an appreciation of the relationship between sleep and muscle pain. This relationship is discussed further in later chapters.
Stages of sleep and bruxing events
Controversy surrounds the stages of sleep during which bruxing occurs. Some studies168,169 suggest that it takes place mainly during the REM stage, while others conclude that bruxism never occurs during REM sleep.170–172 Still other studies173–178 report that bruxing events occur during both REM and non-REM sleep but that most events seem to be associated with the lighter stages (1 and 2), of non-REM sleep. Bruxing events appear to be associated with a change from deeper to lighter sleep, as can be demonstrated by directing a flashing light toward a sleeping person’s face. Such stimulation has been shown to induce tooth grinding.171 The same reaction was observed following sonic and tactile stimulation. Thus this and other studies have indicated that bruxing may be closely associated with the arousal phases of sleep.155,174,175,179,180
Duration of bruxing events
Sleep studies also reveal that the number and duration of bruxing events during sleep vary greatly, not only among persons but also within the same person. Kydd and Daly181 reported that a group of 10 bruxists rhythmically clenched their teeth for a total mean duration of 11.4 min per night. These clenches commonly occurred in single episodes lasting 20 to 40 s each. Reding et al173 reported the average bruxing event as lasting for only 9 s (range 2.7 to 66.5 s), with a total average bruxing time of 40 s/hr. Clarke et al182 reported that bruxing events occurred an average of only five times during an entire sleep period, with an average duration of about 8 s per event. Trenouth183 reported that a TMJ-bruxism group spent 38.7 min with their teeth together during an 8-hr period. In the same study a control group only spent 5.4 min with their teeth together during an 8-hr period. In three separate studies of normal subjects, Okeson et al174–176 found that bruxing events averaged from 5 to 6 s.
There is uncertainty as to the number and duration of bruxing events that can create muscle symptoms. Certainly there is great variation from patient to patient.184 Christensen185–187 demonstrated that pain was produced in subjects’ jaw muscles after 20 to 60 s of voluntary clenching. It would appear, therefore, that bruxing events were able to induce symptoms in some individuals, although the specific nature of the symptoms and how much activity was involved were not reported.
Intensity of bruxing events
The intensity of bruxing events has not been well studied, but C/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses