Chapter 7 Bone Density: A Key Determinant for Treatment Planning
Available bone is particularly important in implant dentistry and describes the external architecture or volume of the edentulous area considered for implants. Historically, the available bone was not modified in the implant candidate. Instead, the existing bone volume was the primary factor used to develop a treatment plan. Short implants and fewer implants were used in less available bone and long implants in greater numbers were inserted in larger bone volumes. Today, the treatment plan first considers the final prosthesis options. The patient force factors are then noted. The next consideration is the bone density in the sites of the implant abutments.
The internal structure of bone is described in terms of quality or density, which reflects a number of biomechanical properties, such as strength and modulus of elasticity. The external and internal architecture of bone controls virtually every facet of the practice of implant dentistry. The density of available bone in an edentulous site is a determining factor in treatment planning, implant design, surgical approach, healing time, and initial progressive bone loading during prosthetic reconstruction.1,2 This chapter presents the aspects of bone density related to overall planning of an implant prosthesis.
INFLUENCE OF BONE DENSITY ON IMPLANT SUCCESS RATES
The quality of bone is often dependent upon the arch position.3–7 The most dense bone is usually observed in the anterior mandible, followed by the anterior maxilla and posterior mandible, and the least dense bone is typically found in the posterior maxilla. Following a standard surgical and prosthetic protocol, Adell et al.8 reported an approximately 10% greater success rate in the anterior mandible as compared with the anterior maxilla. Schnitman et al. also noted lower success rates in the posterior mandible as compared with the anterior mandible when the same protocol was followed.9 The highest clinical failure rates have been reported in the posterior maxilla, where the force magnitude is greater and the bone density is poorer.5–7,9–13 A range of implant survival has been found relative to arch location.
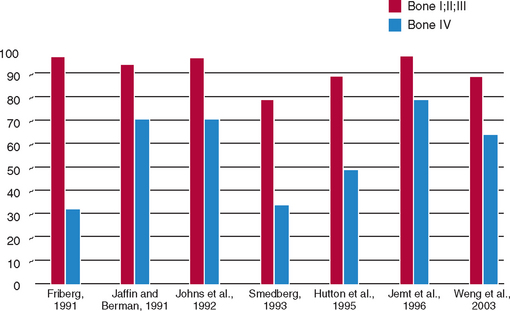
In addition to arch location, several independent groups have reported different failure rates related to the quality of the bone.3–20a Engquist et al. observed that 78% of all reported implant failures were in soft bone types.16 Friberg et al. observed that 66% of their group’s implant failures occurred in the resorbed maxilla with soft bone.3 Jaffin and Berman, in a 5-year study, reported a 44% implant failure when poor-density bone was observed in the maxilla.15 The article documented a 35% implant loss in any region of the mouth when bone density was poor. Fifty-five percent of all implant failures within their study sample occurred in the soft bone type. Johns et al. reported 3% failure of implants in moderate bone densities, but a 28% implant failure in the poorest bone type.17 Smedberg et al. reported a 36% failure rate in the poorest bone density.18 The reduced implant survival most often is more related to bone density than arch location. In a 15-year follow-up study, Snauwaert et al. reported early annual and late failures were more frequently found in the maxilla.12 Hermann et al.13 found implant failures were strongly correlated to patient factors, including bone quality, especially when coupled with poor bone volume (65% of these patients experienced failure). These reported failures are not primarily related to surgery healing, but instead occur after prosthetic loading. Therefore, over the years, many independent clinical groups, following a standardized surgical protocol, documented the indisputable influence of bone density on clinical success (Figure 7-1).*
However, a protocol established by the author, which adapts the treatment plan, implant selection, surgical approach, healing regimen, and initial prosthetic loading, has resulted in similar implant success rates in all bone densities and all arch positions.21–24 This chapter proposes a scientific rationale for the modification of a treatment plan in function of implant density to achieve comparable success rates in all bone types.
ETIOLOGY OF VARIABLE BONE DENSITY
Bone is an organ that is able to change in relation to a number of factors, including hormones, vitamins, and mechanical influences. However, biomechanical parameters, such as duration of edentulous state, are predominant.25–29 Awareness of this adaptability has been reported for more than a century. In 1887, Meier qualitatively described the architecture of trabecular bone in the femur.30 In 1888, Kulmann noticed the similarity between the pattern of trabecular bone in the femur and tension trajectories in construction beams.31 Wolff, in 1892, further elaborated on these concepts and published, “Every change in the form and function of bone or of its function alone is followed by certain definite changes in the internal architecture, and equally definite alteration in its external conformation, in accordance with mathematical laws.”32 The modified function of bone and the definite changes in the internal and external formation of the vertebral skeleton as influenced by mechanical load were reported by Murry.33 Therefore the external architecture of bone changes in relation to function, and the internal bony structure is also modified.
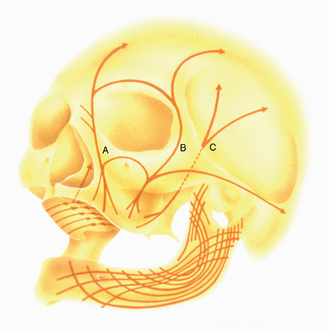
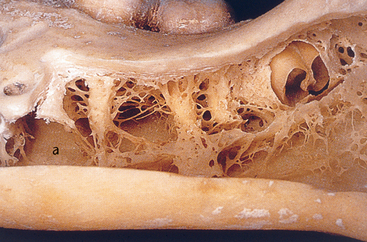
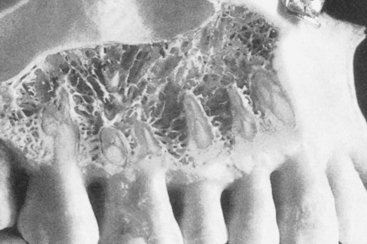
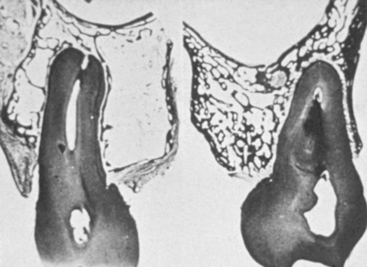
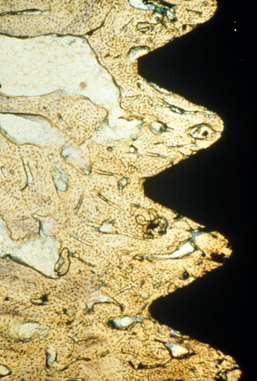
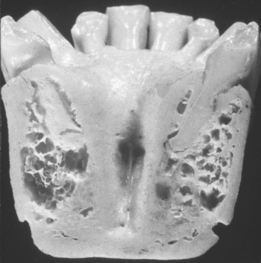
MacMillan34 and Parfitt35 have reported on the structural characteristics and variation of trabeculae in the alveolar regions of the jaws. For example, the maxilla and mandible have different biomechanical functions (Figure 7-2). The mandible, as an independent structure, is designed as a force-absorption unit. Therefore, when teeth are present, the outer cortical bone is denser and thicker and the trabecular bone is more coarse and dense (Figure 7-3). On the other hand, the maxilla is a force-distribution unit. Any strain to the maxilla is transferred by the zygomatic arch and palate away from the brain and orbit. As a consequence, the maxilla has a thin cortical plate and fine trabecular bone supporting the teeth (Figure 7-4). They also noted that the bone is most dense around the teeth (cribriform plate) and more dense around the teeth at the crest, compared with the regions around the apices (Figure 7-5). Alveolar bone resorption associated with orthodontic therapy also illustrates the biomechanical sensitivity of the alveolar processes.36 Generalized trabecular bone loss in the jaws occurs in regions around a tooth from a decrease in mechanical strain.37 Orban demonstrated a decrease in the trabecular bone pattern around a maxillary molar with no opposing occlusion, compared with a tooth with occlusal contacts on the contralateral side38 (Figure 7-6). Bone density in the jaws also decreases after tooth loss. This loss is primarily related to the length of time the region has been edentulous and not loaded appropriately, the initial density of the bone, flexure and torsion in the mandible, and parafunction before and after tooth loss. In general, the density change after tooth loss is greatest in the posterior maxilla and least in the anterior mandible.
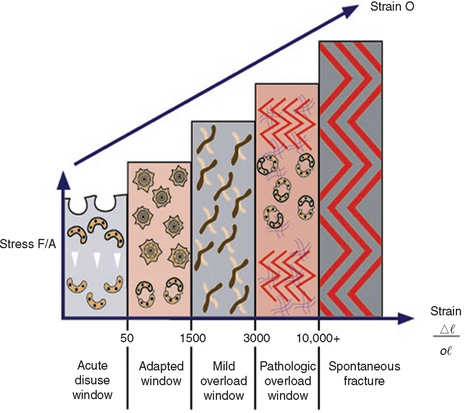
Cortical and trabecular bone throughout the body are constantly modified by either modeling or remodeling.39 Modeling has independent sites of formation and resorption and results in the change of the shape or size of bone. Remodeling is a process of resorption and formation at the same site that replaces previously existing bone and primarily affects the internal turnover of bone, including that region where teeth are lost or the bone next to an endosteal implant.40,41 These adaptive phenomena have been associated with the alteration of the mechanical stress and strain environment within the host bone.42,43 Stress is determined by the magnitude of force divided by the functional area over which it is applied. Strain is defined as the change in length of a material divided by the original length. The greater the magnitude of stress applied to the bone, the greater the strain observed in the bone.44 Bone modeling and remodeling are primarily controlled, in part or whole, by the mechanical environment of strain. Overall, the density of alveolar bone evolves as a result of mechanical deformation from microstrain. Frost proposed a model of four histologic patterns for compact bone as it relates to mechanical adaptation to strain.45 The pathologic overload zone, mild overload zone, adapted window, and acute disuse window were described for bone in relation to the amount of the microstrain experienced (Figure 7-7). These four categories also may be used to describe the trabecular bone response in the jaws.
The bone in the acute disuse window loses mineral density, and disuse atrophy occurs because modeling for new bone is inhibited and remodeling is stimulated, with a gradual net loss of bone. The microstrain of bone for trivial loading is reported to be 0 to 50 microstrain. This phenomenon may occur throughout the skeletal system, as evidenced by a 15% decrease in the cortical plate and extensive trabecular bone loss consequent to immobilized limbs for 3 months.46 A cortical bone density decrease of 40% and a trabecular bone density decrease of 12% also have been reported with disuse of bone.47,48 Interestingly, bone loss similar to disuse atrophy has been associated with microgravity environments in outer space, because the microstrain in bone resulting from the earth’s gravity is not present in the “weightless” environment of space.49 In fact, an astronaut aboard the Russian Mir space station for 111 days lost nearly 12% of his bone minerals.50,51
The adapted window (50 to 1500 microstrain) represents an equilibrium of modeling and remodeling, and bone conditions are maintained at this level. Bone in this strain environment remains in a steady state, and this may be considered the homeostatic window of health. The histologic description of this bone is primarily lamellar or load-bearing bone. Approximately 18% of trabecular bone and 2% to 5% of cortical bone is remodeled each year25 in the physiologic loading zone, which corresponds to the adapted window. This is the range of strain ideally desired around an endosteal implant, once a stress equilibrium has been established (Figure 7-8). Bone turnover is required in the adapted window, as Mori and Burr provide evidence of remodeling in regions of bone microfracture from fatigue damage within the physiologic range.52
The mild overload zone (1500 to 3000 microstrain) causes a greater rate of fatigue microfracture and increase in the cellular turnover rate of bone. As a result, the bone strength and density may eventually decrease. The histologic description of bone in this range is usually woven or repair bone. This may be the state for bone when an endosteal implant is overloaded and the bone interface attempts to change the strain environment. During the repair process, the woven bone is weaker than the more mature, mineralized lamellar bone.40 Therefore, while bone is loaded in the mild overload zone, care must be taken because the “safety range” for bone strength is reduced during the repair.41
Pathologic overload zones are reached when microstrains are greater than 3000 units.45 Cortical bone fractures occur at 10,000 to 20,000 microstrain (1% to 2% deformation). Therefore pathologic overload may begin at microstrain levels of only 20% to 40% of the ultimate strength or physical fracture of cortical bone. The bone may resorb and form fibrous tissue, or when present, repair woven bone in this zone, as a sustained turnover rate is necessary. The marginal bone loss evidenced during implant overloading may be a result of the bone in the pathologic overload zone. Implant failure from overload may also be a result of bone in the pathologic overload zone.
BONE CLASSIFICATION SCHEMES RELATED TO IMPLANT DENTISTRY
An appreciation of bone density and its relation to oral implantology has existed for more than 25 years. Linkow, in 1970, classified bone density into three categories53:
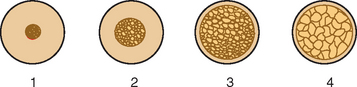
In 1985, Lekholm and Zarb listed four bone qualities found in the anterior regions of the jawbone54 (Figure 7-9). Quality 1 was composed of homogeneous compact bone. Quality 2 had a thick layer of compact bone surrounding a core of dense trabecular bone. Quality 3 had a thin layer of cortical bone surrounding dense trabecular bone of favorable strength. Quality 4 had a thin layer of cortical bone surrounding a core of low-density trabecular bone. Irrespective of the different bone qualities, all bone was treated with the same implant design and standard surgical and prosthetic protocol.8 Following this protocol, Schnitman and others observed a 10% difference in implant survival between Quality 2 and Quality 3 bone, and 22% lower survival in the poorest bone density.3,9,16 Johns et al. reported 3% failure in Type III bone, but 28% in Type IV bone.17 Smedberg et al. reported a 36% failure rate in Type IV bone.18 Higuchi and others also experienced a greater failure in the soft bone of the maxilla.*
It is obvious that a standardized surgical, prosthetic, and implant design protocol does not yield similar results in all bone densities. In addition, these reports are for implant survival, not the quality of health of surviving implants. The amount of crestal bone loss also has been related to bone density,56–60 and further supports a different protocol for soft bone.
In 1988, Misch proposed four bone density groups independent of the regions of the jaws, based on macroscopic cortical and trabecular bone characteristics.1,2 The regions of the jaws with similar densities were often consistent. Suggested treatment plans, implant design, surgical protocol, healing, and progressive loading time spans have been described for each bone density type.23,60,61 Following this regimen, similar implant survival rates have been observed for all bone densities.21–23
MISCH BONE DENSITY CLASSIFICATION
Dense or porous cortical bone is found on the outer surfaces of bone and includes the crest of an edentulous ridge. Coarse and fine trabecular bone types are found within the outer shell of cortical bone and occasionally on the crestal surface of an edentulous residual ridge. These four macroscopic structures of bone may be arranged from the least dense to the most dense, as first described by Roberts and Frost25,45 (Figure 7-10).
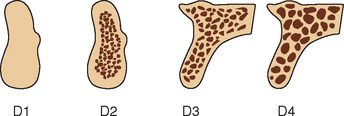
In combination, these four increasing macroscopic densities constitute four bone categories described by Misch (D1, D2, D3, and D4) located in the edentulous areas of the maxilla and mandible (Figure 7-11). The regional locations of the different densities of cortical bone are more consistent than the highly variable trabecular bone.
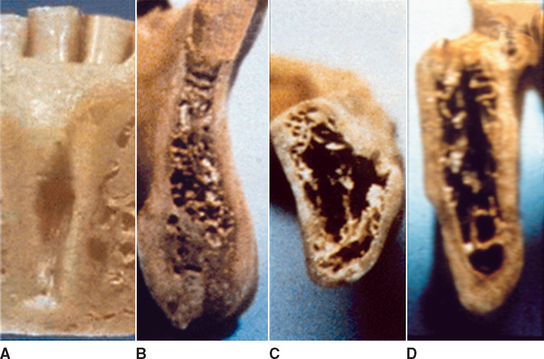
D1 bone is primarily dense cortical bone. D2 bone has dense-to-porous cortical bone on the crest and, within the bone, has coarse trabecular bone. D3 bone types have a thinner porous cortical crest and fine trabecular bone in the region next to the implant. D4 bone has almost no crestal cortical bone. The fine trabecular bone composes almost all of the total volume of bone next to the implant (Table 7-1, Figure 7-12). A very soft bone, with incomplete mineralization and large intertrabecular spaces, may be addressed as D5 bone. This bone type is most often immature bone in a developing sinus graft. The bone density may be determined by tactile sense during surgery, the general location, or radiographic evaluation.
BONE DENSITY LOCATION
A review of the literature and a survey of completely and partially edentulous patients postsurgery indicated that the location of different bone densities often may be superimposed on the different regions of the mouth3–7,11–13,15–20,62–66 (see Tables 7-1 and 7-2). D1 bone is almost never observed in the maxilla and is rarely observed in most mandibles. In the mandible, D1 bone is observed approximately 6% of the time in the Division A anterior mandible and 3% of the time in the posterior mandible, primarily when the implant is engaging the lingual cortical plate of bone. In a C-h bone volume (moderate atrophy) in the anterior mandible, the prevalence of D1 bone approaches 25% in males. The C-h mandible often exhibits an increase in torsion, flexure, or both in the anterior segment between the foramina during function. This increased strain may cause the bone to increase in density. D1 b/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses