Chapter 4 Stress Treatment Theorem for Implant Dentistry
Dentistry is a unique aspect of medicine, blending science and art form. Some aspects of the dental field emphasize the art form, as in dental esthetics, which deals with tooth color and shape to enhance a patient’s smile and overall appearance. However, the primary reason the term doctor is applied to the dental profession is because of the dental sciences. These may be separated into a biological component and a biomechanical component. For general dentists, the biological aspects of oral health are emphasized. Common complications related to the natural dentition are primarily of biological origins, with periodontal diseases, caries, and endodontic problems as examples.1–4
A combination of biological and biomechanical factors is responsible for the failure of tooth-supported fixed prostheses. The four most common complications for three-unit fixed prostheses are (1) caries, (2) endodontic problems, (3) unretained prosthesis, and (4) porcelain fracture.5,6 The biological complications occur with greater frequency (11% to 22%), compared with the biomechanical (7% to 10%), but both aspects should be understood by the clinician.
Implant dentistry always involves the replacement of teeth. When implant complications are reported, the vast majority of problems are related to the implant sciences, rather than esthetics.7 But, unlike natural teeth, the biological aspects of implant dentistry have relatively few complications. For example, the development of a direct bone-implant interface is largely biological. Most recent reports indicate the surgical phase of implants form a successful interface more than 95% of the time, regardless of the implant system used.7 Hence, the biological aspect of the field is very predictable.
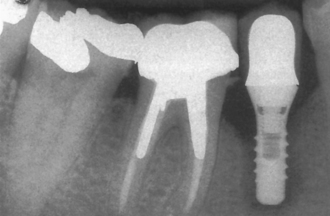
The most common implant-related complications are biomechanical problems that occur after the implant is loaded. A literature review focusing on implant failure indicated these problems primarily occur within 18 months of initial implant loading. These early implant loading failures occur most often in the softest bone types (16% failure) or the shortest implant lengths (17% failure). These two failure groups are typically caused by biomechanical factors. Soft bone is too weak for the occlusal forces applied to the implants, or short implants have higher stresses at the bone-implant interface8–20 (Figure 4-1).
The most common complications that do not lead to the failure of the implant are also biomechanical problems. Implant overdentures have problems of attachment fracture or complication (30%) and removable prosthesis fracture (12%). In implant-supported fixed prostheses, acrylic resin veneer fracture (22%), abutment or prosthetic screw loosening (7% to 10%), porcelain fracture (7%), and prosthesis metal fracture (3%) are examples of common complications.7,15 In addition, implant components (2% to 4%) and even implant bodies may fracture (1% to 2%) (Box 4-1). In other words, mechanical complications far outnumber biological implant problems.7,20
Box 4-1 Stress Treatment Theorem: Biological versus Biomechanical
| Biological | Biomechanical |
|---|---|
| Implant Failure | |
| Surgical failure | Early loading failure |
| Healing | Micromovement |
| Crestal Bone Loss | |
| Periosteal reflection | Cellular biomechanics |
| Osteotomy | Engineering |
| Autoimmune (bacteria) | Bone mechanics |
| Biological microgap | Animal studies |
| Clinical reports | |
| Prosthetic Complications: Mechanics | |
| Screw loosening | Attachment wear |
| Component fracture | Attachment fracture |
| Implant body fracture | Denture tooth fracture |
| Acrylic veneer/porcelain fracture | Acrylic base fracture |
| Framework fracture | Opposing prosthesis fracture |
Any complex engineering structure will fail at its “weakest link,” and dental implant structures are no exception. A general concept in engineering is to determine the causes of complications and develop a system to reduce the conditions that cause the problems. The most common causes for implant-related complications are centered around stress. Thus, the overall treatment plan should (1) assess the greatest force factors in the system and (2) establish mechanisms to protect the overall implant-bone-prosthetic system.
SURGICAL FAILURE
There are many reasons for the failure of an implant to integrate initially with the bone. The primary causes of failure relate to excessive heat during the preparation of the osteotomy or excessive pressure at the implant-bone interface at the time of implant insertion21 (Figure 4-2). The excessive pressure at implant insertion is observed most often with tapered screw-type body designs. The insertion torque force on a tapered screw implant design may place excessive forces on the bone, which leads to resorption and implant failure.
Figure 4-2 Excessive heat during osteotomy preparation can cause surgical failure of a healing implant.
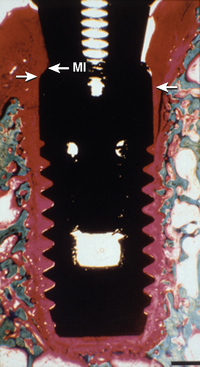
An additional cause of surgical failure is micromovement of the implant while the developing interface is established (Figure 4-3). A fractured arm is immobilized to prevent movement at the fracture site to decrease the risk of a fibrous nonunion. Movement as little as 20 microns has been reported to cause a fibrous interface to form at the fracture site. Brunski observed a fibrous tissue interface development when a dental implant moved more than 100 microns during initial healing.22 The original Brånemark protocol used a two-stage surgical approach.23,24 One of the main reasons for this concept was to place the implant at or below the crestal bone region to decrease the risk of implant movement during initial bone healing. Schroeder also suggested an unloaded healing period on implants, although the implant was placed at or slightly above the gingival tissues.25
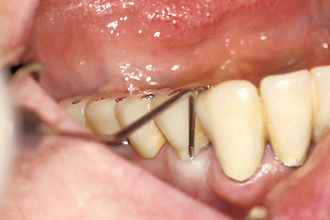
Occlusal forces applied to a removable prosthesis over a healing implant may also cause incision line opening of the soft tissue and delay soft tissue healing.26 These occlusal forces may also affect the marginal bone around the developing implant site. Transferring these forces to an overlying soft tissue-borne prosthesis may cause micromovement of the implant-bone interface, whether the implant is healing below or above the gingival tissues. Stresses applied to a healing implant increase the risk of complications. On the other hand, multicenter clinical reports indicate an experienced surgeon may obtain rigid fixations after surgical placement 99% of the time.27 The surgical component of implant failure is often the least risk associated with the overall implant treatment.
EARLY LOADING FAILURE
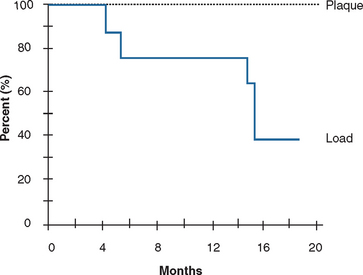
On occasion, an implant may fail shortly after it has initially “integrated” to the bone. Before failure, the implant appears to have rigid fixation, and all clinical indicators are within normal limits. However, once the implant is loaded, the implant becomes mobile within 6 to 18 months (see Figure 4-1). This has been called early loading failure by Misch and Jividen.9 The cause of early loading failure is usually excessive stress for the bone-implant interface. Isidor allowed eight implants to integrate in monkey jaws.28 Crowns were attached to the healed implants with excessive premature occlusal contacts. Over a 20-month period, six of eight implants failed (Figure 4-4). In these same animals, eight integrated implants with no occlusal loads had strings placed in the marginal gingiva to increase the amount of plaque retention. None of these implants failed over the following 20 months. The authors concluded that in this animal model, biomechanical occlusal stress was a greater risk factor for early implant failure than the biological component of bacterial plaque.28,29
Early loading failure is worse for the implant clinician than when a surgical failure occurs, because the patient may blame the restoring dentist. Although this is bad enough, in addition the restoring dentist spent two to five appointments restoring the implant and has a laboratory expense. Early loading failure is related to the amount of force applied to the prosthesis8,22,30–33 and the density of the bone around the implants,7,10–14,34 and it may affect 15% of implant restorations.6–11 Early implant failure from biomechanical overload, as high as 40%, has been reported in the softest bone types.13 No reports in the literature correlate such high incidence extreme with early implant failure rates related to the biological width-related complications observed in the field.
IMPACT OF OCCLUSAL OVERLOAD ON MECHANICAL COMPONENTS
Screw Loosening
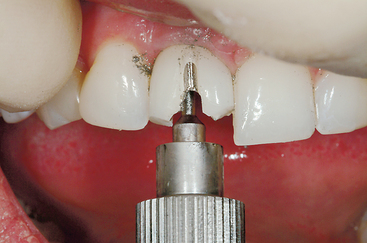
Abutment screw loosening has been detected in an overall average of 6% of implant prostheses.7 Single-tooth crowns exhibited the highest rate of 25% in early screw designs and concepts (and as high as 45%). Recent studies indicate this ratio has been reduced to an overall 8% average, with multiple-unit fixed prostheses at a 5% average and implant overdentures at 3% (Figure 4-5). The greater the stress applied to the prostheses (single tooth versus overdentures), the greater the risk of abutment screw loosening. Cantilevers also increase the risk of screw loosening, as they increase the forces in direct relationship to the length of the cantilever.35 The greater the crown height attached to the abutment, the greater the force applied to the screw, and the greater the risk of screw loosening.
The height or depth of an antirotational component of the implant body also can affect the amount of the force applied to the abutment screw. The higher (or deeper) the hex height, the less stress applied to the screw and a corresponding lower risk for abutment screw loosening.35,36 The platform dimension upon which the abutment is seated is even more important than the hex height dimension. Larger-diameter implants, with larger platform dimensions, reduce the forces applied to an abutment screw and change the arc of displacement of the abutment on the crest module. Screw loosening also may be decreased by a preload force with a torque wrench on the screw. Therefore, prosthetic methods to decrease stress to the abutment screw or engineering approaches to decrease stress or increase thread tightening may be used to decrease the incidence of complications related to screw loosening.
Fatigue Fractures
Prosthesis screw fracture has been noted in both fixed partial and complete fixed prostheses, with a mean incidence of 4% and a range of 0% to 19%7 (Figure 4-6). Abutment screws are usually larger in diameter and therefore fracture less often, with a mean incidence of 2% and a range of 0.2% to 8% (Figure 4-7). Metal framework fractures also have been reported in an average of 3% of fixed complete and overdenture restorations, with a range of 0% to 27% (Figure 4-8). Implant body fracture has the least incidence of this type of complication, with an occurrence of 1% (Figure 4-9). This condition is reported with more frequency in long-term fixed prostheses and may even account for the majority of long-term failures. Resin veneer fractures of fixed implant prostheses averaged 22%, overdentures clip/attachment fractures averaged 17%, porcelain veneer fractures averaged 14%, overdenture fractures averaged 12%, and acrylic-base resin fractures averaged 7%. Prostheses-related fractures far outnumber implant component fractures.
Figure 4-8 Metal framework fracture has been reported with an average of 3% with implant-fixed prostheses.
Uncemented restorations (or worse, partially uncemented prostheses) occur most likely when chronic loads are applied to the cement interface, or when shear forces are present (as found with cantilevers). Cement strengths are weakest in shear loads. Zinc phosphate cement may resist a compressive force of 12,000 psi but can only resist a shear force of 500 psi. It is interesting to note that bone is also strongest to compression and 65% weaker to shear forces. A similar scenario relative to shear load is found with porcelain or other occlusal materials. As a consequence, the evaluation, diagnosis, and modification of treatment plans related to stress conditions are of considerable importance. Therefore, once the implant dentist has identified the sources of additional force on the implant system, the treatment plan is altered in an attempt to minimize their negative impact on the longevity of the implant, bone, and final restoration.
MARGINAL BONE LOSS
Crestal bone loss has been observed around the permucosal portion of dental implants for decades. It has been described in the crestal region of successfully osteointegrated implants regardless of surgical approaches. It can range from loss of marginal bone to complete failure of the implant17,23,37,38 and dramatically decreases after the first year (Box 4-2). For the one-piece blade implants, this phenomenon was described as a “saucerization” and occurred after implant loading.37
Occlusal Trauma: Bone Loss
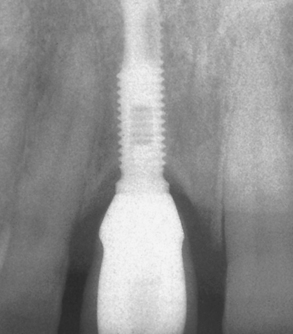
Adell et al.23 were the first to quantify and report marginal bone loss. The study also indicated greater magnitude and occurrence of bone loss during the first year of prosthesis loading, averaging 1.2 mm during this time frame, with a range of 0 to 3 mm. This report measured bone loss from the first thread as the 0-mm baseline, not from the original level of crestal bone at insertion, which was 1.8 mm above this baseline point. Thus the actual first-year crestal bone loss averaged 3.3 mm around the implants observed (Figure 4-10). Years subsequent to the first showed an average of 0.05 to 0.13 mm bone loss per year. Other studies report an average first-year bone loss of 0.93 mm, with a range from 0.4 to 1.6 mm and a mean loss of 0.1 mm after the first year.31,32 The early crestal bone loss has been observed so frequently that proposed criteria for successful implants often do not even include the first-year bone loss amount.39
The initial transosteal bone loss around an implant forms a V- or a U-shaped pattern, which has been described as ditching or saucerization around the implant. The current hypotheses for the cause of crestal bone loss have ranged from reflection of the periosteum during surgery, preparation of the implant osteotomy, the position of the microgap between the abutment and implant body, micromovement of the abutment components, bacterial invasion, the establishment of a biological width, and factors of stress.23,17,38–42
An understanding of the causes of marginal crestal bone loss around dental implants and early implant failure is critical in preventing such occurrences, fostering long-term periimplant health, and improving long-term implant success rates and, foremost, implant prosthesis success. Marginal crestal bone loss may influence esthetics, as the height of the soft tissue (e.g., interdental papilla) is directly related to the marginal bone. If the tissue shrinks as a consequence of the bone loss, the emergence profile of the crown elongates and the papilla may disappear next to the adjacent tooth or implant. If the soft tissue does not shrink, the increase in pocket depth may be related to the presence of anaerobic bacteria and peri-implantitis.
Periosteal Reflection Hypothesis
Periosteal reflection causes a transitional change in the blood supply to the crestal cortical bone. Ninety percent of the arterial blood supply and 100% of the venous return are associated with the periosteum in the long bones of the body.43 When the periosteum is reflected off the crestal bone, the cortical bone blood supply is affected dramatically, causing osteoblast death on the surface from trauma and lack of nutrition. These events have fostered the periosteal reflection theory as a cause for early bone loss around an endosteal implant.
Although crestal bone cells may die from the initial trauma of periosteal reflection, the blood supply is reestablished once the periosteum regenerates. Cutting cones develop from monocytes in the blood and precede new blood vessels into the crestal regions of bone. Osteoblasts then are able to remodel the crestal bone anatomy.44 Composite bone forms rapidly on periosteal surfaces to restore its original condition. In addition, the underlying trabecular bone is also a vascular source because its blood supply often is maintained in spite of crestal periosteal reflection. The greater the amount of trabecular bone under the crestal cortical bone, the less crestal bone loss is observed.45 To place the implant in sufficient available bone, an implant ridge is usually 5 mm or wider at the crest. As a result, trabecular bone is readily available to assist in cortical blood supply and remodeling around the implants. The cortical bone is remodeled to its original contour, without significant loss of height.
The periosteal reflection theory would lead to a generalized horizontal bone loss of the entire residual ridge reflected, not the localized ditching pattern around the implant that typically is observed. In addition, generalized bone loss already would be directly noticeable at the second-stage uncovery of the implant body, 4 to 8 months after Stage I implant placement surgery. Yet generalized bone loss rarely is observed at the second-stage uncovery surgery (Figure 4-11). Therefore, the periosteal reflection hypothesis does not appear as a primary causal agent of marginal crestal bone loss around an implant.
Implant Osteotomy Hypothesis
Preparation of the implant osteotomy has been reported as a causal agent of early implant bone loss. Bone is a labile organ and is sensitive to heat. The implant osteotomy causes trauma to the bone in immediate contact with the implant, and a devitalized bone zone of about 1 mm is created around the implant. A renewed blood supply and cutting cones are necessary to remodel the bone at the interface. The crestal region is more susceptible to bone loss during initial repair because of its limited blood supply and the greater heat generated in this denser bone, especially with the less efficient cutting of countersink drills used in this region.45–47 This condition supports implant osteotomy preparation as a causal agent for marginal crestal bone loss around the implant.
However, if heat and trauma during implant osteotomy preparation were responsible for marginal crestal bone loss, the effect would be noticeable at the second-stage uncovery surgery 4 to 8 months later. The average bone loss of 1.5 mm from the first thread is not observed at Stage II uncovery. In fact, bone often has grown over the first-stage cover screw, especially when level or slightly countersunk below the bone (see Figure 4-11). Reports in the literature indicate different surgical trauma causes and numbers for bone loss. For example, Manz48 observed that bone loss at second-stage surgery ranged from 0.89 to 0.96 mm regardless of the bone density. Hoar et al.49 reported only 0.2-mm bone loss at Stage II uncovery. The surgical system or approach may influence these data, but usually this bone loss remains minimal.
Autoimmune Response of Host Hypothesis
The primary cause of bone loss around natural teeth is bacteria induced. Repeat studies demonstrate that bacteria are the causative element for vertical defects around teeth. Occlusal trauma may accelerate the process, but trauma alone is not deemed a determining factor.50 The implant gingival sulcus in the partially edentulous implant patient exhibits a bacterial flora similar to that of natural teeth.1 A logical assumption is that if implants are similar to teeth, the marginal implant bone loss is caused primarily by bacteria, with occlusal factors playing a contributing or accelerating role.
In a prospective study of 125 implants, Adell et al.38 reported 80% of implant sulcular regions were without inflammation. Lekholm et al.51 found that deep gingival pockets around implants were not associated with crestal bone loss. Yet the marginal crestal bone loss to the first thread of screw-type implants is a common radiologic finding. If bacteria were the causal agent for the initial bone loss, why does most bone loss occur the first year (1.5 mm) and less (0.1 mm) each successive year? The implant sulcus depth progressively increases from the early bone loss, impairing hygiene and making anaerobic bacteria more likely as the cause of bacteria-related bone loss. If bacteria are responsible for 1.5-mm early crestal bone loss, what local environmental changes occur to reduce their effect by 15 times after the first year?23 The bacteria autoimmune theory cannot explain the marginal bone loss condition when it follows the pattern most often reported.
Although the bacteria theory does not explain adequately the marginal crestal bone loss phenomenon, this does not mean that bacteria are not a major contributor to bone loss around an implant. Threads and porous implant surfaces exposed to bacteria are reported to cause a more rapid loss of bone around an implant.51 Poor hygiene also is reported to accelerate the bone loss observed around endosteal implants52,53 (Figure 4-12). To state that bacteria are never involved in marginal bone loss around an implant would be incorrect. Bone loss often is associated with bacteria as a causal agent. However, when most bone loss occurs in the first year and less bone loss is observed afterward, the hypothesis of bacteria as the primary causal agent for the early crestal bone loss cannot be substantiated.
Biological Width Hypothesis
The sulcular regions around an implant and around a tooth are similar in many respects. The rete peg formation within the attached gingiva and the histologic lining of the gingiva within the sulcus are similar in implants and teeth. A free gingival margin forms around an implant with nonkeratinized sulcular epithelium, and the epithelial cells at its base are similar to the functional epithelial cells described with natural teeth.54 However, a fundamental difference characterizes the base of the gingival sulcus.
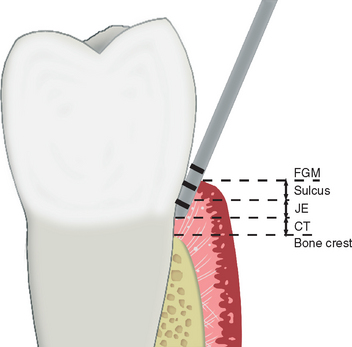
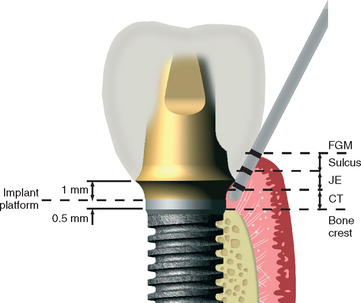
For a natural tooth, an average biological width of 2.04 mm exists between the depth of the sulcus and the crest of the alveolar bone (Figure 4-13). It should be noted the biological “width” is actually a height dimension with a greater range in the posterior region compared with the anterior, and may be greater than 4 mm in height. In teeth, it is composed of a connective tissue (CT) attachment (1.07 mm average) above the bone and a junctional epithelial attachment (0.97 mm average) at the sulcus base, with the most consistent value between individuals being the CT attachment.55–57 The biological width allows gingival fibers and hemidesmosomes to establish direct contact with the natural tooth and acts as a barrier to the bacteria in the sulcus to the underlining periodontal tissues. When a crown margin invades the biological width, the crestal bone recedes to reestablish a favorable environment for the gingival fibers.58,59
Many surgical protocols recommend the placement of endosteal implants at or below the crest of the ridge during the first-stage surgery. The abutment-to-implant body connection may be compared with a crown margin. Berglundh et al.60 observed 0.5 mm of bone loss below the implant-abutment connection within 2 weeks after stage II uncovery and abutment connection in dogs (Figure 4-14). Lindhe et al.61 reported an inflammatory connective tissue extending 0.5 mm above and below this implant abutment connection. Wallace and Tarnow62,63 stated that the biological width also occurs with implants and may contribute to some of the marginal bone loss observed. The biological width theory seems attractive to explain the lack of bone loss from the first stage of surgery and the early bone loss seen within the first year after the second-stage abutment placement. However, it should be noted that the biological “width” in implants, as reported, often includes the sulcus depth, whereas the natural tooth biological width does not include the sulcus depth.
Eleven different gingival fiber groups are observed around a natural tooth: dentogingival (coronal, horizontal, and apical), alveologingival, intercapillary, transgingival, circular, semicircular, dentoperiosteal, transseptal, periosteogingival, intercircular, and intergingival. At least six of these gingival fiber groups insert into the cementum of the natural tooth: the dentogingival (coronal, horizontal, and apical), dentoperiosteal, transseptal, circular, semicircular, and transgingival fibers. In addition, some crestal fibers from the periodontal fiber bundles also insert into the cementum above the alveolar bone.57 However, in a typical implant gingival region, only two of these gingival fiber groups and no periodontal fibers are present (Figure 4-15). These fibers do not insert into the implant body below the abutment margin as they do into the cementum of natural teeth.56,64 Instead, the collagen fibers in the CT attachment around an implant run parallel to the implant surface, not perpendicular, as with natural teeth.65,66 The gingival and periosteal fiber groups are responsible for the connective tissue attachment component of the biological width around teeth, and these are not present around the transosteal region of an implant. Therefore the CT attachment around the abutment-implant connection cannot be compared with the CT attachment of a tooth.
James and Keller64 were first to begin a systematic scientific study to investigate the biological seal phenomenon of the soft tissue around dental implants. Hemidesmosomes help form a basal lamina—like structure on the implant, which can act as a biological seal. However, collagenous components of the linear body cannot physiologically adhere to or become embedded into the implant body as they do in the cementum of the tooth.67 The hemidesmosomal seal only has a circumferential band of gingival tissue to provide mechanical protection against tearing.68 Therefore the biological seal around dental implants can prevent the migration of bacteria and endotoxins into the underlying bone. It is unable, however, to constitute a junctional epithelial attachment component of the biological width similar to the one found with natural teeth. The amount of early crestal bone loss therefore seems unlikely to be solely the result of the remodeling of the hard and soft tissues to establish a biological width below an abutment connection. No connective tissue attachment zone or components of the linear body are embedded into an implant. The importance, amount, and mechanism for these anatomical structures require further investigation.
The crevice between the cover screw and the implant body during initial healing is similar to the crevice of the abutment-implant connection. Yet bone can grow over the cover screw, and therefore the crevice, in and of itself, may not be the cause of bone loss. The crevice between the implant and the abutment connection has been called a “microgap.” The actual dimension of this connection is usually 0 μm and has a direct metal to metal connection. However, when the crevice is exposed to the oral environment, bone loss is usually observed for at least 0.5 mm below the connection.68–70
The primary question remains, when the surgeon places the implant abutment connection below the bone, how much bone loss is from the implant biological width, and therefore out of the influence of the dental practitioner? Several reports in the literature note implant macro- and microgeometry may affect the biological width dimensions or the amount of early crestal bone loss.31,32,49,71–74
Occlusal Trauma
Marginal bone loss on an implant may be from occlusal trauma.40 Occlusal trauma may be defined as an injury to the attachment apparatus as a result of excessive occlusal force.1 A controversy exists as to the role of occlusion in the bone loss observed after an implant prosthesis delivery.8 Some articles state that peri-implant bone loss without implant failure is primarily associated with biological formations or complications.16–18 Other authors suggest a correlation of crestal bone loss to occlusal overload.8,40,41,75,76 The determination of the etiology of bone loss around dental implants is needed in order to minimize its occurrence and foster long-term peri-implant health that may ultimately determine implant p/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses