4 Potential for Infection
4.0 INTRODUCTION
The immune system is the primary defense mechanism against potential pathogens, including bacteria, viruses, fungi, and parasites. To prevent infection, the body possesses a complex, layered defense system that begins with physical barriers to foreign insult (e.g., skin, mucous). If a pathogen breaches these physical barriers, activation of the innate immune system occurs, triggering an immediate, non-specific response by leukocytes (white blood cells) including macrophages, neutrophils, and dendritic cells as well as antimicrobial proteins such as lysozyme, defensins, and the complement system. Next, activation of the adaptive or acquired immune system generates a pathogen-specific humoral (B-cell) and cell-mediated (T-cell) immune response that results in long-term memory of specific pathogens. Normal immune response is dependent on leukocytes, and alterations in the number or function of these cells may render a patient susceptible to infection.
Oral health care providers manage dental diseases and are therefore responsible for preventing and/or eliminating bacterial infections of both pulpal and periodontal etiology. Further, prior to performing invasive dental treatment, the oral health care provider must consider a patient’s risk for bacterial infection to determine whether the procedure is safe and/or necessary, and whether prophylactic and/or post-operative measures are warranted. This chapter will discuss evaluation of patient risk for oral bacterial infection associated with dental treatment; however, these principles generally apply to oral viral and fungal infections as well (see chapter 7).
4.1 Medical conditions associated with an increased risk of oral infection
A number of medical conditions are characterized by quantitative and/or qualitative compromise of the immune system. In addition to the disorders discussed in this chapter, other conditions warrant consideration, such as autoimmune and immune-mediated conditions, which may be associated with an increased risk of oral infection primarily due to the secondary effects of immunosuppressive therapies (see chapter 11). Further, a number of other rare disorders such as cyclic neutropenia and common variable immune deficiency (CVID) are associated with an increased risk of infection.
Figure 4.1 Gingival infiltrate of leukemic cells in a 42-year-old female recently diagnosed with acute lymphocytic leukemia.
4.1.1 Hematologic malignancies
Patients with hematologic malignancies (e.g., leukemia and lymphoma) are at a significantly increased risk of developing infections due to primary and secondary immunosuppression. Hematologic malignancies are characterized by clonal proliferation and dysfunction of a specific hematopoietic cell line. The bone marrow, lymphatic system, peripheral circulation, and major organs may be involved, and foci of malignant cells may also be present in the oral cavity (Figure 4.1). Overproduction of malignant cells may lead to deficits in normal-functioning leukocytes and in some cases cytopenias (reduction of other blood cells). Management of hematologic malignancies typically includes high-dose intensive chemotherapy with or without hematopoietic stem cell transplantation (HSCT), which also significantly increases the risk of infection (see chapter 10).
4.1.1.1 Leukemia
Leukemia is characterized by an abnormal proliferation of white blood cells (WBCs). This disease can affect almost any age group, although some subtypes are more prevalent later in life (e.g., chronic lymphocytic leukemia), while others affect predominantly children (e.g., acute lymphoblastic leukemia). Leukemias are further subdivided by cell lineage. Lymphoblastic or lymphocytic leukemias (acute lymphoblastic leukemia, chronic lymphocytic leukemia) affect the bone marrow cells that differentiate into lymphocytes (B-cells and T-cells), while myelogenous leukemias (acute myelogenous leukemia, chronic myelogenous leukemia) affect the bone marrow cells that differentiate into red blood cells, platelets, and other types of WBCs. Leukemias are characterized by an abnormally elevated white blood cell count, though these cells are highly dysfunctional and therefore patients are at significant risk for infection. In addition, thrombocytopenia (decreased platelet count) may occur, resulting in an increased risk of bleeding (see chapter 3).
Treatment for leukemia involves high-dose induction chemotherapy followed by multiple sessions of consolidation chemotherapy, although the specific regimen and sequence varies depending on the underlying diagnosis (see chapter 10). Periods of significant and in some cases prolonged myelosuppression are expected during treatment (Section 4.2.1). Depending on the specific leukemia diagnosis and response to initial chemotherapy, patients may undergo HSCT, potentially resulting in profound immunosuppression and very high risk for infection during and following transplantation.
4.1.1.2 Lymphoma
Lymphoma is a malignant proliferation of lymphocytes that presents as solid tumors. Lymph nodes are most commonly affected, resulting in lymphadenopathy and complications secondary to bulky disease, but lymphoma can also occur in other locations, including the oral cavity (extranodal lymphoma). The World Health Organization categorizes lymphomas by cell type into the following groups: (a) Hodgkin lymphoma, (b) B-cell neoplasms, (c) T-cell and natural killer (NK) cell neoplasms, and (d) immunodeficiency-associated lymphoproliferative disorders. Hodgkin lymphoma is characterized by the proliferation of mature, dysfunctional B-cells and primarily affects adults, with peaks in the second and eighth decades of life. Non-Hodgkin lymphomas are characterized by malignant proliferation of B-lymphocytes (85% of cases) or T-lymphocytes or NK cells (15% of cases). Lymphoma or other lymphoproliferative disorders may also be associated with conditions characterized by immunosuppression, such as human immunodeficiency virus (HIV) infection or organ transplant recipients.
Chemotherapy is the mainstay of treatment, with occasional use of adjuvant radiotherapy. Patients may also be treated with hematopoietic stem cell transplantation (see chapter 10).
4.1.1.3 Multiple myeloma
Multiple myeloma is a malignancy of clonal plasma cells that secrete elevated amounts of immunoglobulin (antibodies) or fragments of immunoglobulin. Malignant cells proliferate in bones, where they cause lytic lesions and bone pain and increase the risk for pathologic fractures. Multiple myeloma is more common in males and African-Americans, with median survival ranging from 4 to 7 years. Increased risk of infection results from reduced hematopoiesis as well as decreased production and increased destruction of normal antibodies. Other common complications include hypercalcemia, renal failure, thrombocytopenia, and anemia. In addition to multiagent chemotherapy protocols and autologous HSCT, high-dose intravenous antiresorptive therapy is a mainstay of myeloma management. Antiresorptive agents are intended to prevent skeletal fractures but have been associated with osteonecrosis of the jaw and can lead to secondary infectious complications (see chapter 10).
4.1.2 Aplastic anemia
Aplastic anemia is an acquired or inherited hematologic deficiency affecting all marrow lineages. Aplastic anemia manifests clinically as pancytopenia, with decreases in WBC, red blood cell, and platelet counts (see chapter 2). Risk of infection and bleeding is increased proportionally to the cell counts. Aplastic anemia is largely idiopathic but has been associated with a number of etiologic factors including environmental exposure to chemicals or ionizing radiation, medications, autoimmune disease, and genetic syndromes (e.g., dyskeratosis congenita). Treatment for aplastic anemia includes immunosuppressive therapy, targeted immune therapy, androgen therapy, and, in severe cases, allogeneic HSCT.
Table 4.1 Oral manifestations of HIV disease.
| Infectious | Viral – HSV and VZV: recurrent mucosal/skin eruption – HPV: mucosal papillomas – CMV-associated ulceration – EBV: oral hairy leukoplakia Fungal (candidiasis, deep fungal: histoplasmosis, coccidiomycosis) Bacterial (acute necrotizing gingivitis/periodontitis) |
| Neoplastic | Non-Hodgkin lymphoma Squamous cell carcinoma Kaposi sarcoma |
| Immune-mediated | Diffuse parotid enlargement Major aphthous ulcers |
4.1.3 HIV infection
Human immunodeficiency virus (HIV) was estimated to affect thirty-four million people worldwide in 2010. The target for HIV is the CD4+ T-cell, with viremia gradually depleting CD4+ T-cells, weakening the immune system, and rendering patients susceptible to life-threatening opportunistic infections. Acquired immunodeficiency syndrome (AIDS) is defined by the World Health Organization as documented HIV infection in combination with a CD4+ T-cell count below 200 cells/mm3, a CD4+ T-cell percentage less than 15%, or an AIDS-defining illness (e.g., deep fungal infection, HIV-encephalopathy, lymphoma, disseminated tuberculosis). The diagnosis of HIV is performed using a serum-based enzyme-linked immunoassay (ELISA) and confirmatory Western blot. An FDA-approved salivary-based rapid test for infection is available to all health care providers and may provide a simple alternative for screening for HIV disease. This test can be utilized in outpatient settings for high-risk individuals, and when positive, confirmatory serum testing is recommended due to its higher specificity.
Management of HIV infection is based on a combination of nucleoside and non-nucleoside reverse transcriptase inhibitors, protease inhibitors, cellular entry blockers, and integrase inhibitors. With the inception of highly active antiretroviral therapy (HAART) in the mid-1990s, HIV disease for those with access to therapy has become a chronic and largely manageable disease. HIV disease monitoring is based on periodic (at least once every 6 months) testing for CD4+ T-cell count, CD8+ T-cell count, and HIV viral load. Decreased CD4+ T-cell and abnormal CD4+/CD8+ counts are markers for disease progression, and a decreased CD4+ T-cell count below 500 cells/mm3 indicates significant immune dysfunction and increased risk for infection. Measurements of viral load may also be utilized to assess disease progression or response to treatment. Viral load is determined by measuring plasma levels of HIV RNA, with detectable copy numbers indicating response or progression based on an individual’s previous numbers and overall trends.
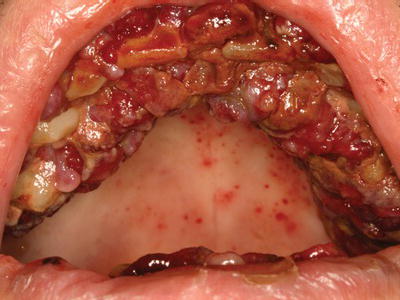
There are numerous infectious and non-infectious oral manifestations of HIV disease, and the presence of these conditions may signify disease progression or inadequate response to therapy (Table 4.1). Acute necrotizing periodontitis occurs with increased frequency in patients with HIV disease and is characterized by generalized gingival ulceration and necrosis, pain, and halitosis (Figure 4.2). Oral candidiasis is the most common opportunistic infection in HIV-positive patients (see chapter 7). Deep fungal infections, such as Aspergillus or Coccidioides immitis, develop infrequently in the oral cavity, typically presenting as a solitary ulceration in severely immunocompromised patients. Herpes virus infections are relatively common in HIV-positive patients. Recrudescent orofacial herpes simplex virus (HSV) infection presents as herpes labialis or as intraoral ulcerative lesions (see chapter 7). Reactivation of varicella zoster virus (VZV) and cytomegalovirus (CMV) intraorally or extraorally occurs much less frequently and may correlate with disease progression. Oral hairy leukoplakia is an Epstein-Barr virus (EBV)–associated condition characterized by bilateral asymptomatic white plaques on the ventrolateral surfaces of the tongue (see chapter 7). Kaposi sarcoma is a neoplasm associated with human herpes virus 8 and frequently presents as intraoral pigmented plaques and nodular lesions (Figure 4.3). Squamous papillomas are caused by human papilloma virus (HPV) infection and may present in multiple areas throughout the oral cavity (Figure 4.4). Recurrent major aphthous ulcers can be quite severe and are highly associated with a suppressed CD4+ T-cell count.
Figure 4.2 Acute necrotizing ulcerative periodontitis in a 37-year-old AIDS patient with a CD4+ T-cell count of 2 cells/mm3.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


