4 Lasers in Surgical Periodontics
Advantages of Laser Surgery
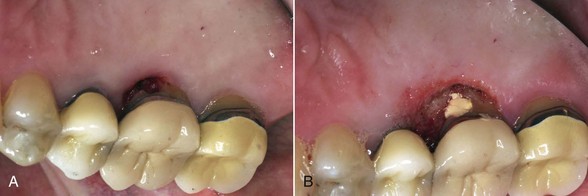
Although laser settings can be a major factor in wound healing, if the clinician respects tissue by using the optimal settings for the given procedure, laser therapy will result in equal or accelerated wound healing (Figure 4-1). Studies on wound healing with lasers (e.g., Nd:YAG, diode, CO2) versus the scalpel generally show an initial lag in soft tissue wound healing but equivalence within 2 weeks of postoperative observation.1,2 Many of these studies were done more than a decade ago with units that emitted much higher fluences than current lasers. Studies performed with more modern equipment by more knowledgeable researchers might show equivalence between scalpel and laser wound healing within 24 hours of the incision.
The results obtained must be discussed on a wavelength-by-wavelength basis; results of carbon dioxide (CO2) wound studies cannot be extrapolated to Nd:YAG, diode, or erbium wavelengths. As with all “hi-tech” devices and instruments, the results depend greatly on receiving the best training possible (see Chapter 16) and knowing how to adjust the watts (W), millijoules (mJ), hertz (Hz), duty cycle, pulsewidth, hand speed, and other parameters (see Chapter 2).
Many laser procedures avoid extensive flap reflection and significant trauma to the wound area. Therefore, with minimal invasive site preparation, the inflammatory response is decreased, resulting in greater patient comfort. Decreased pain and swelling results from laser sealing of the lymphatics and nerve endings.3,4 Biostimulation of the wound area and enhanced wound healing may also occur5,6 (see Chapter 15).
The neodymium-doped yttrium-aluminum-garnet (Nd:YAG) and diode lasers emit wavelengths that are absorbed more readily in pigmented tissues, such as tissues with a high concentration of hemoglobin. Use of these lasers therefore creates a hemostatic environment during and immediately after surgery as the hemoglobin absorbs the laser energy.7 CO2 lasers create hemostasis through a different mechanism. When collagen in the walls of the blood vessels absorbs the CO2 wavelength, the helical collagen polymer unravels. This results in shrinkage of the collagen fibers, causing the lumens of the blood vessels to shrink, creating hemostasis.3
Therefore, significant hemostasis can be achieved in laser procedures, especially in medically compromised patients (Figure 4-2). Current medical histories reflect extensive pharmaceutical intervention to anticoagulate patients, including prophylactic aspirin and platelet aggregation inhibitors. Patients may also be using many “natural” homeopathic remedies and other preparations that may interfere with clotting. They may not include the use of these over-the-counter (OTC), nonprescription preparations in their history. Such compounds as curry powder, cayenne pepper, cinnamon, and other herbs and spices are high in salicylate content and can affect clotting. Ginkgo biloba, vitamin E, and other preparations available in health food stores and pharmacies also can affect clotting.
Some superficial soft tissue procedures can be performed with commercially available topical anesthetics, such as those containing lidocaine and prilocaine. Orthodontic and pediatric patients requiring frenectomy and gingivoplasty or gingivectomy are especially suitable candidates for laser procedures with topical anesthesia (see Chapters 12 and 13).
Nonsurgical Applications
Most laser therapy does have antimicrobial properties. Nd:YAG and diode lasers are absorbed by bacteria, especially those with pigmentation, therefore reducing colonization.8,9 Decreasing bacterial load in the soft tissue wound site can enhance wound healing, with less postoperative discomfort. CO2 and erbium laser wavelengths are absorbed by the water content in cells, causing cell vaporization when the temperature of the intracellular water exceeds 100° C.
Although the use of lasers in initial periodontal therapy has significant advantages, it must be emphasized that laser utilization is an adjunct to standard therapy rather than a replacement therapy. For example, when the clinician considers standard nonsurgical scaling and root planing, the goals include reduction of the bacterial biofilm, removal of necrotic cementum and subgingival calculus, and de-epithelialization of the sulcus. Scaling and root planing should result in decreased inflammation, decreased pocket depth, and attachment gain through a long junctional epithelial attachment.10 Lasers do not remove necrotic cementum or subgingival calculus but do aid in reduction of the biofilm and de-epithelialize tissue more quickly and easily than conventional techniques. Rossmann et al.11 emphasize that CO2 lasers can be used to delay the apical downgrowth of epithelium, and that this technique is less technically demanding and more time-efficient than other techniques.
The Nd:YAG and diode lasers have limited use in hard tissue root therapy as root detoxification because they are primarily soft tissue lasers. In contrast, erbium lasers demonstrate the capacity for root debridement by their effect on calculus and necrotic cementum, with reduced endotoxins.12,13 Also, evidence indicates that these effects can increase the attachment level gain over scaling and root planing.14,15 However, systematic reviews (especially evidence based) demonstrate minimal differences in nonsurgical periodontal end points between laser and conventional periodontal therapy. Evidence shows that soft tissue curettage does not contribute to additional gains in attachment level versus meticulous periodontal root planing in chronic adult periodontitis. Therefore, soft tissue lasers such as the Nd:YAG and diode, with their ability to de-epithelialize the gingival sulcus and some antibacterial properties, may have limited application for nonsurgical periodontal therapy. The erbium laser, in addition to soft tissue use, may be the device required for calculus removal and hard tissue detoxification, creating a biocompatible surface for attachment of connective or epithelial tissue.16 Using the CO2 wavelength, Crespi et al.17 found they could increase the quality and quantity of fibroblasts attaching to the root surface.
As an adjunct to periodontal debridement, photodynamic therapy may have potential. Whether using a “cold” (low-level) laser or a conventional dental laser absorbed by pigment (e.g., diode, Nd:YAG), some clinicians use methylene blue dye as a subgingival irrigant and place it in the sulcus. These laser wavelengths are attracted to and interact with the dye, disrupting the bacterial cell membranes. The light energy activates the dye, interacts with intracellular oxygen, and destroys the bacteria by lipid peroxidation and membrane damage. Chapters 3 and 15 discuss the use of lasers for initial (nonsurgical) periodontal therapy.
Gingivectomy
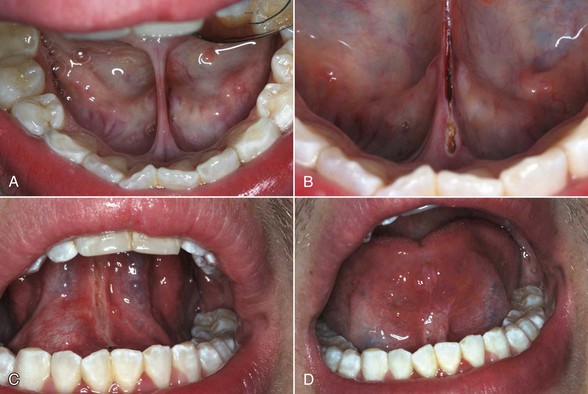
The gingivectomy is a time-honored procedure for removal of gingiva. The indications range from access to esthetics. The gingivectomy can be used when suprabony pockets are present and access to osseous structures is not necessarily important. The procedure assists in decreasing gingival enlargement and altering fibrotic gingiva (Figure 4-3). However, gingivectomy is contraindicated when (1) access to osseous structure is critical or (2) attached gingiva is inadequate (minimal) or absent.
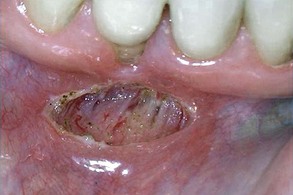
The laser generally demonstrates delayed epithelialization, collagen production, and inflammation with a lower tensile strength. In later wound healing, however, the laser wound accelerates, with collagen production and epithelialization. There are fewer myofibroblasts present during healing of a laser-resected wound site, which leads to less wound contraction and less scar formation18 (Figure 4-4).
As discussed earlier, there is no clear conclusion on healing rates of laser-induced wounds versus conventional scalpel wounds. However, Nd:YAG, CO2, erbium-doped YAG (Er:YAG), and diode lasers demonstrate wound healing that is either comparable to conventional scalpel blades or somewhat accelerated.19 White et al.20 compared several laser technologies using histological analysis and determined that wound healing is based on watts, hertz, pulse duration, and time of exposure. Therefore, it could be concluded that wound healing with a flap or a gingivectomy procedure using laser therapy depends as much on the settings of the device as the actual laser wavelength. Also, training is often as important as or sometimes more important than laser wavelength.
An alternative to laser therapy, electrosurgery (especially monopolar devices) does not have a defined target tissue as laser technology does. The primary mode of tissue interaction with electrosurgical instruments is by heat ablation. The zone of necrosis after electrosurgery can be 500 to 1500 µ (µm). Diode and Nd:YAG lasers can generate heat in tissue up to 500 µ, whereas erbium and CO2 lasers, because of high water absorption, penetrate from 5 to 40 µ. Bipolar electrosurgery units are an improvement over the monopolar units in that bipolar units generate less lateral heat and can be used in a wet environment.21
Treating noninflamed, fibrotic gingiva with diode and Nd:YAG lasers requires different power settings than hyperemic or vascular tissue.22 Lasers are attracted by specific chromophores, so less power is needed to incise tissue if there is a great amount of the chromphore in the tissue. When the gingiva is hyperemic and inflamed, less power is needed because of the high amount of chromophore (hemoglobin) in the tissue. More power is needed to incise fibrotic tissue with less chromophore (hemoglobin) present. The same holds true for gingivectomies on patients with different melanin content. Melanin is one of the chromophores of Nd:YAG and diode lasers. Tissue that is heavily pigmented with melanin requires much less power than gingiva that is very light, coral pink.
The clinician must appreciate both the emission spectrum of the laser and the absorption spectrum of the tissue. What wavelength is being emitted? What is the primary chromophore of the tissue? How does tissue biotype affect the laser parameters? All the variables of laser use (power, spot size, pulse per second, and hand speed) must be taken into account, along with wavelength and tissue biotype (see Chapter 2). It is a mistake to consider that increasing power alone may result in quicker cutting through the target tissue. Lasers generate heat that may result in tissue necrosis from lateral thermal damage. Therefore, settings are critical in performing laser therapy. Again, it must be emphasized that training is one of the primary considerations when evaluating the different laser manufacturers before purchase.
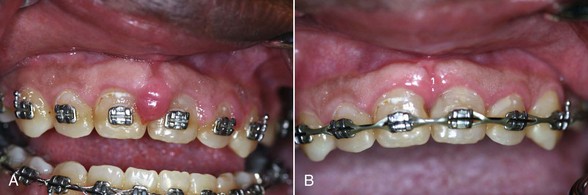
Because of the sculpting ability of a laser procedure, gingivoplasty can now be performed to smooth the gingival margins with a parabolic appearance. With diode, CO2, and Nd:YAG lasers, hemostasis is achieved during the procedure. Erbium lasers create hemostasis after the procedure by altering laser parameters to seal blood vessels. Again, some clinicians incorporate the erbium laser “bandage” as the final procedure. Whether or not to place a dressing is a clinician preference (Figure 4-5).
Frenectomy
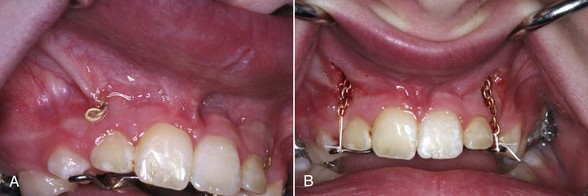
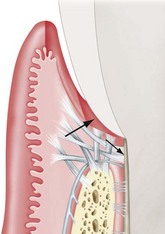
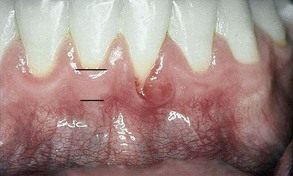
The use of the frenectomy procedure in periodontics (unlike orthodontic/pediatric considerations) is limited because of minimal increase in attached gingivae after postfrenectomy wound healing (Figure 4-6). Although alveolar mucosa is characterized by a red color and smooth surface and is generally loose and mobile, attached gingiva is keratinized, pink, stippled, and firm with no mobility (Figure 4-7).
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses