1 Einstein’s “Splendid Light”
Origins and Dental Applications
The medicinal use of light for diagnostic and therapeutic purposes dates from antiquity. Light allowed early physicians to observe skin color, inspect wounds, and choose a suitable therapeutic course of action. Heat from sunlight or campfires was used for therapy. Greeks and Romans took daily sunbaths, and solariums were incorporated in many Roman houses.1 Ancient Egyptians, Chinese, and Indians used light to treat rickets, psoriasis, skin cancer, and even psychosis.2
The ancient Egyptians, Indians, and Greeks also used natural sunlight to repigment patients with vitiligo by activating the naturally occurring photosensitizer psoralen, found in parsley and other plants.3–5 In the eighteenth and nineteenth centuries, European physicians used sunlight and artificial light to treat cutaneous tuberculosis, psoriasis, eczema, and mycosis fungoides.3 These and other applications of light were precursors to the invention and subsequent use of a special form of light—lasers—in the medical field over the past 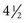 decades.
decades.
Early Published Theories of Light
In his Book of Optics, published in 1021, Persian mathematician, scientist, and philosopher Ibn al-Haytham described light as being composed of a stream of tiny particles that travel in straight lines and bounce off objects that they strike.6 Pierre Gassendi, a French philosopher, scientist, astronomer, and mathematician, described his particle theory of light (published posthumously in 1658 in Lyon, France, as part of the six volumes of his collected works, the Opera Omnia), in effect introducing to European scholars the atomism view of the universe identified by the ancient Greek philosopher Epicurus (341-270 bce)7 (Figure 1-1).
Gassendi’s work influenced English physicist Sir Isaac Newton (1642-1727), who described light as “corpuscles” or particles of matter that “were emitted in all directions from a source”8,9 (Figure 1-2). Newton proposed the theory of particle dynamics, which later would be developed to describe the behavior of particles reacting to the influence of arbitrary forces.10 The particle view of light differed from that of French philosopher and scientist René Descartes, who in his 1637 Discourse saw light as a type of “pressure,” which foreshadowed the postulation of the wave theory of light11 (Figure 1-3).
In 1665, English scientist Robert Hooke suggested his wave theory of light, likening the spread of light vibrations to that of waves in water: “every pulse or vitration of the luminous body will generate a sphere, which will continually increase, and grow bigger, just after the same manner (though infinitely swifter) as the waves or rings on the surface of the water do swell into bigger and bigger circles about a point.”12 The wave concept was subsequently proved experimentally by Scottish physicist James Clerk Maxwell, who in 1865 proposed an electromagnetic wave theory of light and demonstrated that electromagnetic waves traveled at precisely the speed of light.13
Development of Quantum Theory
On Dec. 14, 1900, German physicist Max Planck delivered a lecture before the German Physical Society (Deutsche Physikalische Gesellschaft) in which he theorized that light consisted of discrete and indivisible packets of radiant energy that he named quanta. He described what eventually became known as the elemental unit of energy (E), as E = hv, where h is a constant of nature with the dimension of action (= energy × time, 6.626 × 10−34 joule second), subsequently called Planck’s constant, and v is the frequency of radiation. Planck’s theory was published late in 1900.14–16 Eleven years later, British physicist Ernest Rutherford contributed to quantum theory when he postulated a planetary model of the atom based on his experimental observations of the scattering of alpha particles by atoms. In his view an atom comprises a central charge surrounded by a distribution of electrons orbiting within a sphere.17
Danish physicist Niels Bohr synthesized Rutherford’s atom model with Planck’s quantum hypothesis (Figure 1-4). In a series of papers published in 1913, Bohr proposed a theory in which electrons revolve in specific orbits around a nucleus without emitting radiant energy. He described the stable, “ground state” of an atom, when all its electrons are at their lowest energy level. Bohr also theorized that an electron may suddenly jump from one specific orbital level to a higher level; to do so, an electron must gain energy. Conversely, an electron must lose energy to move from a higher energy level to a lower level. Thus, an electron can move from one energy level to another by either absorbing or emitting radiant energy or light.18,19
It was in this burgeoning milieu of nascent quantum theory that Albert Einstein made three significant contributions. First, in 1905, Einstein developed his light quantum theory: “In the propagation of a light ray emitted from a point source, the energy is not distributed continuously over ever-increasing volumes of space, but consists of a finite number of energy quanta localized at points of space that move without dividing, and can be absorbed or generated as complete units.”20 Singh21 points out that this paper on photoelectric effect was the first that Einstein published during his annus mirablis (extraordinary year), in the scientific journal Annalen der Physik in 1905; his other papers that year treated Brownian motion, special theory of relativity, and matter and energy equivalence (E = mc2). Notably, Einstein himself regarded his light quantum paper as the “most revolutionary” of those that he had published in 1905. He was awarded the 1921 Nobel Prize in physics for this paper. Hallmark and Horn22 state that Einstein’s light quantum theory was so radical compared with other contemporary theories of light that it was not generally accepted until American physicist Robert A. Millikan performed additional experiments in 1916 to support the theory.
Einstein’s 1905 paper made the case for the particle nature of light. In 1909, Einstein made his second significant contribution to laser theory by publishing the first reference in physics to the wave-particle duality of light radiation, using Planck’s radiation law. Einstein stated: “It is my opinion that the next phase in the development of theoretical physics will bring us a theory of light which can be interpreted as a kind of fusion of the wave and emission theory. … Wave structure and quantum structure … are not to be considered as mutually incompatible. … We will have to modify our current theories, not to abandon them completely.”21,23 In 1916-1917, Einstein made his third important contribution to laser theory by providing a new derivation of Planck’s radiation law,24–26 with vast implications. As he wrote to his friend Michele Angelo Besso in 1916, “A splendid light has dawned on me about the absorption and emission of radiation.”21 Indeed, his new idea provided the basis for subsequent laser development.
Based on quantum theory, two fundamental radiation processes associated with light and matter were known before Einstein’s new derivation: (1) stimulated absorption, a process in which an atom can be excited to a higher energy state through such means as heating, light interaction, or particle interaction; and (2) spontaneous emission, the process of an excited atom decaying to a lower energy state spontaneously, by itself. Einstein’s breakthrough was the addition of a third alternative: stimulated emission, the reverse of the stimulated absorption process. In the presence of other incoming radiation of the same frequency, excited atoms are stimulated to make a transition to the lower energy state—more quickly than in spontaneous emission—and in the process release light energy identical to the incoming form of light. The emitted light has the same frequency and is in phase (i.e., coherent) with the stimulating radiation wave. Stimulated emission occurs when there are more excited atoms than atoms that are not excited (i.e., more atoms in upper of two energy levels than in lower level), a condition called population inversion. Einstein also showed that the process of stimulated emission occurs with the same probability as absorption from the lower state.27–30 Hey et al.31 summarized the significance of Einstein’s insight as follows:
At this point, it should be clarified that the term photon was not used by Planck, Bohr, or Einstein up to the time of the Einstein’s 1916-17 papers. American chemist Gilbert Lewis32 was apparently the first to use the term when he argued, in a letter to the editor of Nature magazine in 1926, for the need for new nomenclature to describe discrete units of radiant energy:
The following accepted definition of photon appears in the American Heritage Dictionary33:
Decades followed Einstein’s 1916-17 articles on stimulated emission before significant progress was made in laser development, both theoretically and practically, in the 1950s and 1960s, partly because of the contemporary outlook and training of physicists at the time, as suggested by American physicist Arthur L. Schawlow and later observers. Schooled in the idea that “thermodynamic equilibrium,” a state of energy balance, was the normal condition of matter throughout the universe, they tended to believe that population inversion was merely an unusual event or brief permutation, not something particularly significant.34,35
However, the 1920s and 1930s were not entirely bereft of discovery and insight. In 1928, German physicist Rudolf Ladenburg indirectly observed stimulated emission while studying the optical properties of neon gas at wavelengths near a transition where the gas absorbed and emitted light. This was the first evidence that stimulated emission existed.34,36 In his 1939 doctoral dissertation, Soviet physicist Valentin A. Fabrikant had envisioned a way to produce a population inversion, writing that “such a ratio of populations is in principle attainable. … Under such conditions we would obtain a radiation output greater than the incident radiation.”34,35,37
Masers and Lasers
The impact of this situation on laser development is exemplified by the work of American physicist Charles H. Townes at Bell Telephone Laboratories in Manhattan and later at Columbia University, which he joined in 1948 (Figure 1-5). In 1941, Townes was assigned to work on a military radar project. Modern radar, a system of using transmitted and reflected radio waves for detecting a reflected object to determine its direction, distance, height, or speed, was developed in the 1930s, when systems used radio waves about a meter long and could not discern much detail. During the war, the military was interested in developing a radar system that used much higher radio frequencies to attain greater sensitivity, tighter radio beams, and transmitting antennas small enough to fit on an airplane. Townes began working on microwave frequencies of 3, 10, and 24 gigahertz (GHz).34 Although none of these systems was used in battle, his experience with the 24-GHz system, interest in microwave spectroscopy, and use of surplus equipment guided Townes toward subsequent development.

FIGURE 1-5 • Charles H. Townes with a ruby microwave maser amplifier developed for radio astronomy in 1957.
(Courtesy Alcatel-Lucent USA.)
In 1951, at the spring meeting of the American Physical Society in Washington, DC, Townes proposed the concept of a maser, an acronym he and his students coined for microwave amplification by stimulated emission of radiation. He indicated that the “primary object of the work that led to the maser was to get shorter wavelengths so we could do better spectroscopy in a new spectral region.”38 Townes elaborated on April 26, 1951: “I sketched out and calculated requirements for a molecular-beam system to separate high-energy molecules from lower[-energy] ones and send them through a cavity which would contain the electromagnetic radiation [photons] to stimulate further emission from the molecules, thus providing feedback and continuous oscillation.”39 On May 11, Townes sketched the idea in his laboratory notebook, dated it, and signed it “Chas. H. Townes.” In February 1952, his colleague and brother-in-law Arthur L. Schawlow also signed the page.34,35
On his return to Columbia University after the April 1951 conference, Townes and postdoctoral fellow Herbert J. Zeiger and doctoral student James P. Gordon commenced work on building a maser. They began to experiment with a beam of ammonia molecules, a compound familiar to Townes from his work on the 24-GHz radar system. It was known that ammonia molecules absorb microwaves at a frequency of 24 GHz, causing the nitrogen atom of that molecule to vibrate. Initial success was achieved in late 1953, when Gordon saw evidence of stimulated emission and amplification from their device; then, in early April 1954, they achieved the desired oscillation.34 They reported their success in a late paper at a meeting of the American Physical Society on May 1, then in a short paper in Physical Review.40
While on sabbatical from Columbia University in 1955, Townes worked with French physicist Alfred Kastler at the École Normale Supérieure in Paris. Kastler developed the technique of “optical pumping,” a process in which light is used to raise (or pump) electrons from a lower to a higher energy level, as a new way to excite materials for microwave spectroscopy.34 Townes recognized that optical pumping might excite the optical energy levels necessary for an optical maser. In fall 1957, Townes and Schawlow, a postdoctoral fellow under Townes at Columbia until he joined Bell Labs in 1951, proposed extending maser principles to the infrared and visible region of the electromagnetic spectrum.35,38 They subsequently published their influential paper in Physical Review in 1958.41
Meanwhile, another American physicist, Gordon Gould, a Columbia graduate student in 1957, asked whether optical pumping could excite light emission. He recorded his ideas in nine handwritten pages of a laboratory notebook, with the first page titled “Some rough calculations on the feasibility of a LASER: Light Amplification by Stimulated Emission of Radiation,” the first time the term laser was used. Gould had his notes notarized on Nov. 13, 1957, which he saw as a necessary step in applying for a patent. His patent defense efforts were finally recognized after 30 years of delays, challenges, and litigation.34,38,42
The Schawlow and Townes paper stirred a number of organizations to conduct additional research into optical masers, as follows34:
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses