Chapter 4
Glass-ionomer Cements
Aim
The aim of this chapter is to provide information on the different types of glass-ionomer cements available and the unique properties of this group of materials.
Outcome
Readers will better comprehend how the composition and setting reaction of glass-ionomer cement dictates its handling to optimise clinical performance, including fluoride release, bond strength and dimensional stability.
Introduction
Glass-ionomer cement was first made commercially available in 1976 as a self-adhesive, tooth-coloured filling material called ASPA. It derived its name as an acronym of the major constituents, aluminosilicate glass and polyacrylic acid. The early materials were slow-setting and difficult to handle, with relatively poor aesthetics. Subsequently, there have been major improvements in the properties of this important group of materials.
Indications
Modern glass-ionomer cement is a versatile, “smart” dental material, with the following applications:
-
definitive restorative material in low load-bearing areas in adults
-
definitive restorative material for deciduous teeth
-
provisional restorative material in adults
-
core build-up material prior to crown placement
-
liners and base
-
luting cement for crowns, posts and bridges
-
fissure sealant
-
bonding agent for composite resins and dental amalgam.
Advantages
Glass-ionomer cements are popular materials as they display the following clinical advantages:
-
they are tooth-coloured
-
they bond chemically to tooth substance and non-precious metals without the need for additional adhesives
-
they release fluoride
-
their coefficient of thermal expansion is equivalent to that of tooth structure
-
they have good biocompatibility.
Disadvantages
The advantages of glass-ionomer cements are offset by the following disadvantages:
-
low fracture toughness, limiting applications in high load-bearing areas
-
some types cannot be finished and polished at the same visit they are placed
-
some types are vulnerable to acid erosion
-
some types exhibit low flexural strength and wear resistance.
Types of Glass-ionomer Cement
Three main types of glass-ionomer cement are commonly used. They have different compositions and properties. These types are:
-
conventional glass-ionomer cement
-
conventional, high-viscosity, reinforced glass-ionomer cements (Fig 4-1)
-
resin-modified glass-ionomer cements (Figs 4-2 and 4-3).
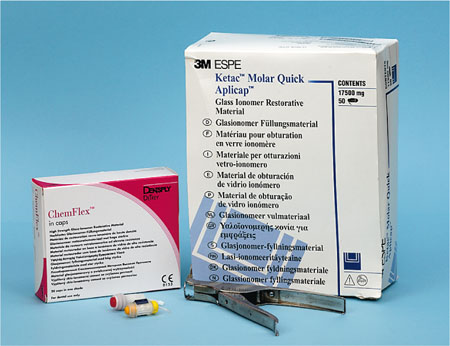
Fig 4-1 Examples of conventional high-viscosity or reinforced glass-ionomer cement, presented in capsules to be activated and mixed in an amalgamator.
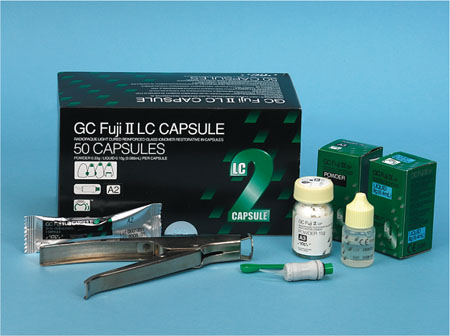
Fig 4-2 A resin-modified glass-ionomer cement, provided in both hand-mixed and capsulated versions.
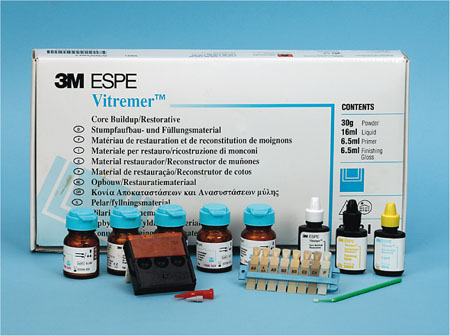
Fig 4-3 A resin-modified glass-ionomer cement, showing the range of shades available, powder:liquid formulation, conditioner and finishing gloss.
Conventional Glass-ionomer Cements
Glass Powders
Conventional glass-ionomer cements consist of an alkaline (basic) aluminosilicate glass with fluoride, which reacts with an acidic poly(alkenoic) acid to create a salt matrix and water. The glass filler particles are predominantly calcium aluminosilicate glasses, but certain manufacturers replace some of the calcium with strontium or lanthanum to increase cement radiopacity. An increase in radiopacity makes it easier for the clinician to identify the presence of recurrent caries under a restoration in a radiograph. Many different types of glasses are used, but the essential formulae are:
SiO2–Al2O3–CaF2 or SiO2–Al2O3–CaO
The glasses receive heat treatment during manufacture. This treatment alters surface reactivity of the powder, as does the particle size. A reduction in particle size increases reactivity, giving improved physical properties. Traditional glass-ionomer cements contained particles of up to 45 μm diameter. In modern materials this has been reduced to 1–15.5 μm.
Fluoride is present in the glass powder as calcium and sodium fluoride. Initially, it was added as a flux and to improve handling properties. The presence of the fluoride ion contributes to the formation of complex bodies with the metallic ions, released in to the liquid during the setting reaction. CaF+ and AlF2+ are formed, which delay the bonding of the metallic cations with either polyacrylic acid – to form calcium and aluminium polyacrylate – or with the COO– groups in the copolymer chains. This has the effect of slowing initial setting by gelation and thereby the working time is lengthened. Fluoride represents approximately 20% of the final glass powder. When the cement is mixed and set, the majority of the fluoride is released from the newly formed salt matrices.
Polyalkenoate Acid
The liquids are high molecular weight electrolytes based on homopolymers of acrylic acid or copolymers with itaconic or maleic acids. Copolymers were developed to improve the shelf life of the liquid, which had a tendency to become too viscous and inappropriate for use after about six months storage. The physical properties of a glass-ionomer cement can be improved by increasing the molecular weight or the concentration of the polyalkenoate acid.
Benefits attained are, however, limited by the fact that the cement becomes too viscous to be clinically useful above certain levels. Some manufacturers have mitigated the effects of this by freeze-drying the acid and adding it as a component in the powder, to be mixed with water alone or a mixture of water and tartaric acid.
Tartaric Acid
The addition of 5–10% of optically active L-tartaric acid improved the handling properties of the cement by delaying initial setting, similar to the action of fluoride, and then providing a rapid onset reaction. The clinical benefits of this are that it gives the clinician longer to manipulate the cement into the cavity and place a matrix if required, whilst shortening the length of time required for the material to set. In addition it increases the compressive strength of the cement (Nicholson, 1998).
Water
Water is an essential component of glass-ionomer cement. This distinguishes glass-ionomer cement from the majority of other tooth-coloured restorative materials, which are polymer based and hydrophobic. Between 11 and 24% of the set glass-ionomer cement is water, some “loosely” bound, some “tightly” bound. The loosely bound water is easily lost if the relative humidity surrounding a newly placed restoration falls below 70%. This is clinically critical, because if the cement is allowed to dehydrate, the loosely held water is lost very rapidly by evaporation, leading to excessive shrinkage. This shrinkage causes the cement to crack, compromising aesthetics and the physical properties of the cement. Cement dehydration is most likely to occur if the cement is isolated under a rubber dam, or is finished or polished with rotary instruments without the application of water coolant (see Chapter 7, pages 109–110). Slower setting aesthetic glass-ionomer cements are vulnerable to dehydration for up to six months after placement, while the faster-setting materials are less vulnerable after two weeks.
Glass-ionomer cement may also be damaged if exposed to excess water early after mixing and placement. Calcium and aluminium cations required for the setting reaction (see below) can be eluted in the presence of excess water, which interferes with the setting reaction, producing weak, unaesthetic cement with a chalky surface. Therefore, the surface of newly placed glass-ionomer cement must be protected from damage by saliva or premature mouth rinsing. Covering the setting cement with a matrix and isolating the tooth with cotton wool rolls, together with low-volume suction, most easily achieves this. Immediately the matrix is removed, a protective layer of low-viscosity methacrylate-based resin sealant or surface gloss should be applied to the surface of the cement and light-cured (Fig 4-4). Alternative materials for this use include petroleum jelly, copal or other proprietary varnishes, but they have a tendency to become porous and, as a consequence, relatively ineffective.

Fig 4-4 Example of a finishing gloss to be applied to the surface of a newly placed conventional glass-ionomer cement to prevent dehydration-induced structural damage.
Setting Reaction
The setting reaction of conventional glass-ionomer cement is shown in Fig 4-5. When the powder and liquid are mixed together the acid goes into solution. H+ ions are released, which react with the outer layer of the fluoroaluminosilicate glass, releasing calcium, aluminium, sodium and fluoride ions. This is called the “dissolution phase”. The loss of the ions converts the outer layer of the glass particles into a siliceous gel. The calcium ions are released most rapidly and form calcium polyacrylate salt, initiating the setting reaction, known as the “gelation phase”. At this stage the cement is very vulnerable to loss of calcium and aluminium ions if exposed to excess water, and it must therefore be kept isolated. It is also important that the powder and liquid are rapidly mixed and placed in the cavit/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


