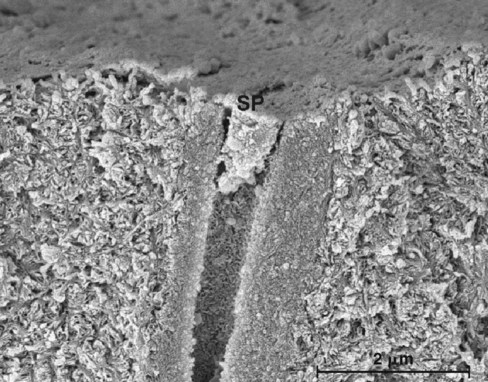
Fundamental Concepts of Enamel and Dentin Adhesion
Basic Concepts of Adhesion
The American Society for Testing and Materials (specification D 907) defines adhesion as “the state in which two surfaces are held together by interfacial forces which may consist of valence forces or interlocking forces or both.”1 The word adhesion comes from the Latin adhaerere (“to stick to”). An adhesive is a material, frequently a viscous fluid, that joins two substrates together by solidifying and transferring a load from one surface to the other. Adhesion or adhesive strength is the measure of the load-bearing capacity of an adhesive joint.2 Four different mechanisms of adhesion have been described, as follows:3
1. Mechanical adhesion—interlocking of the adhesive with irregularities in the surface of the substrate, or adherend
2. Adsorption adhesion—chemical bonding between the adhesive and the adherend; the forces involved may be primary (ionic and covalent) or secondary (hydrogen bonds, dipole interaction, or van der Waals) valence forces
3. Diffusion adhesion—interlocking between mobile molecules, such as the adhesion of two polymers through diffusion of polymer chain ends across an interface
4. Electrostatic adhesion—an electrical double layer at the interface of a metal with a polymer that is part of the total bonding mechanism
In dentistry, bonding of resin-based materials to tooth structure is a result of four possible mechanisms, as follows:4
1. Mechanical—penetration of resin and formation of resin tags within the tooth surface
2. Adsorption—chemical bonding to the inorganic component (hydroxyapatite) or organic components (mainly type I collagen) of tooth structure
3. Diffusion—precipitation of substances on the tooth surfaces to which resin monomers can bond mechanically or chemically
A major problem in bonding resins to tooth structure is that all methacrylate-based dental resins shrink during free-radical addition polymerization.5 Dental adhesives must provide a strong initial bond to resist the stresses of resin shrinkage.
Trends in Restorative Dentistry
The introduction of enamel bonding, the increasing demand for restorative and nonrestorative esthetic treatments, and the ubiquity of fluoride have combined to transform the practice of operative dentistry.6,7 The classic concepts of tooth preparation were advocated in the early 1900s;8 but these have changed drastically. This transformation in philosophy has resulted in a more conservative approach to tooth preparation, with regard to not only the basic concepts of retention form but also the resistance form of the remaining tooth structure. Bonding techniques allow more conservative tooth preparations, less reliance on macromechanical retention, and less removal of unsupported enamel.
1. Restore Class I, II, III, IV, V, and VI carious or traumatic defects
2. Change the shape and the color of anterior teeth (e.g., with full or partial resin veneers)
3. Improve retention for porcelain-fused-to-metal (ceramometal) or metallic crowns
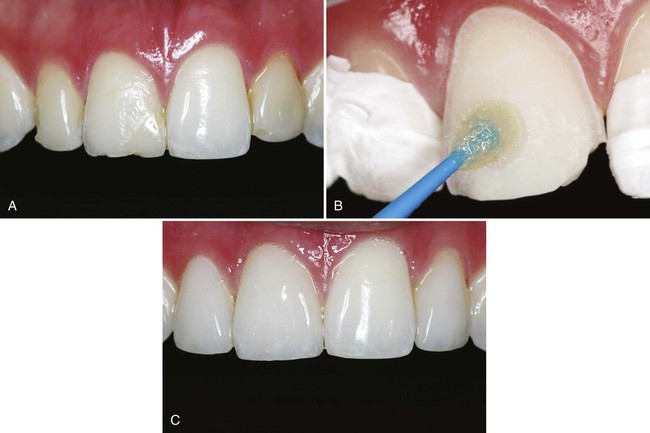
4. Bond all-ceramic restorations (Fig. 4-1)
7. Bond periodontal splints and conservative tooth-replacement prostheses
8. Repair existing restorations (composite, amalgam, ceramic, or ceramometal)
9. Provide foundations for crowns
10. Desensitize exposed root surfaces
11. Seal beneath or bond amalgam restorations to tooth structure
12. Impregnate dentin that has been exposed to the oral fluids, making it less susceptible to caries
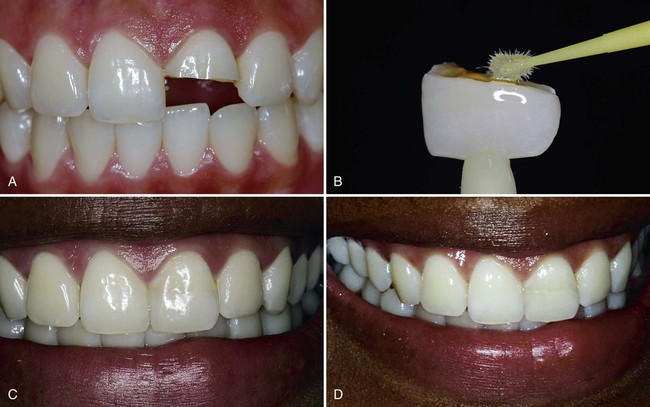
13. Bond fractured fragments of anterior teeth (Fig. 4-2)
14. Bond prefabricated fiber or metal posts and cast posts
15. Reinforce fragile endodontically treated roots internally
16. Seal root canals during endodontic therapy
17. Seal apical restorations placed during endodontic surgery
Enamel Adhesion
Inspired by the industrial use of 85% phosphoric acid to facilitate adhesion of paints and resins to metallic surfaces, Buonocore envisioned the use of acids to etch enamel for sealing pits and fissures.6 Since Buonocore’s introduction of the acid-etch technique, many dental researchers have attempted to achieve methods for reliable and durable adhesion between resins and tooth structure.
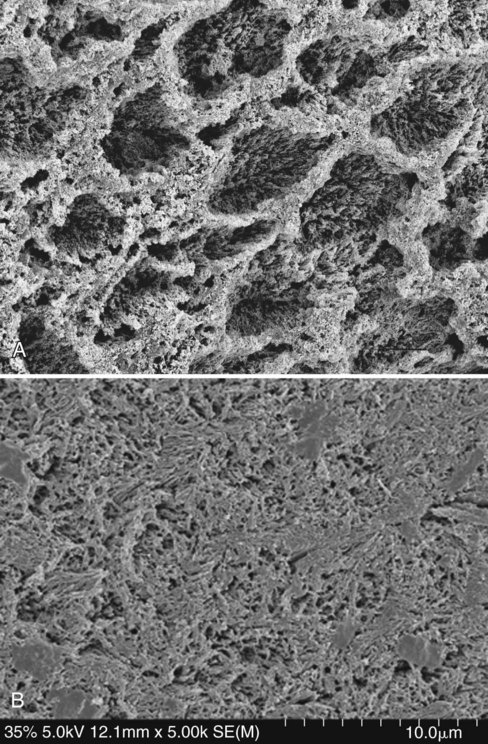
Acid-etching transforms the smooth enamel into an irregular surface (Fig. 4-3) and increases its surface free energy. When a fluid resin-based material is applied to the irregular etched surface, the resin penetrates into the surface, aided by capillary action. Monomers in the material polymerize, and the material becomes interlocked with the enamel surface (Fig. 4-4).9,10 The formation of resin microtags within the enamel surface is the fundamental mechanism of resin-enamel adhesion.10–12 Figure 4-5 shows a replica of an etched enamel surface visualized through the extensions of resin that penetrated the irregular enamel surface. The acid-etch technique has revolutionized the practice of restorative dentistry.

Enamel etching results in three different micromorphologic patterns.13,14 The type I pattern involves the dissolution of prism cores without dissolution of prism peripheries (Fig. 4-6, A). The type II etching pattern is the opposite of type I: the peripheral enamel is dissolved, but the cores are left intact (see Fig. 4-3). Type III etching is less distinct than the other two patterns. It includes areas that resemble the other patterns and areas whose topography is not related to enamel prism morphology (see Fig. 4-6, B).
Beginning with Buonocore’s use of 85% phosphoric acid, various concentrations of phosphoric acid have been used to etch enamel. Most current phosphoric acid gels have concentrations of 30% to 40%, with 37% being the most common, although some studies using lower concentrations have reported similar adhesion values.15–17
An etching time of 60 seconds originally was recommended for permanent enamel using 30% to 40% phosphoric acid. Although one study concluded that shorter etch times resulted in lower bond strengths, other studies using scanning electron microscopy (SEM) showed that a 15-second etch resulted in a similar surface roughness as that provided by a 60-second etch.11,18–20 Other in vitro studies have shown similar bond strengths and leakage for etching times of 15 and 60 seconds.21–25
As measured in the laboratory, shear bond strengths of composite to phosphoric acid-etched enamel usually exceed 20 megapascals (MPa) and can range up to over 50 MPa, depending on the test method used.26–29 Such bond strengths provide adequate retention for a broad variety of procedures and prevent leakage around enamel margins of restorations.24
Dentin Adhesion
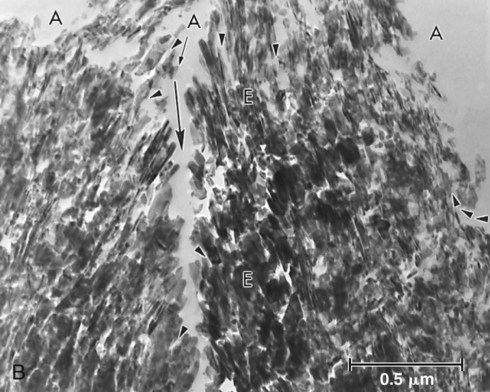
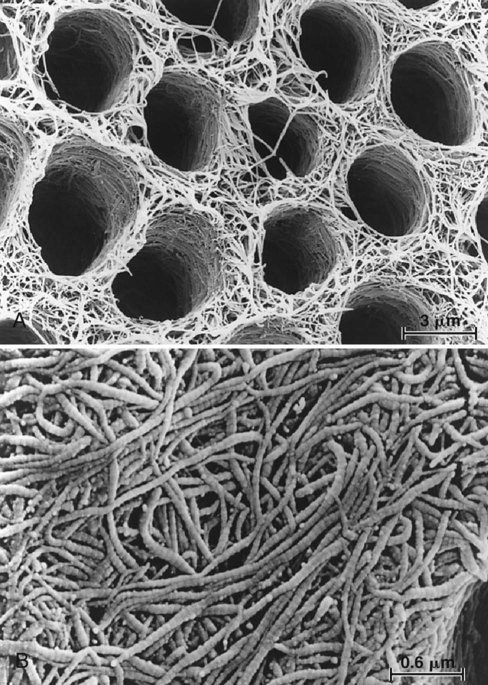
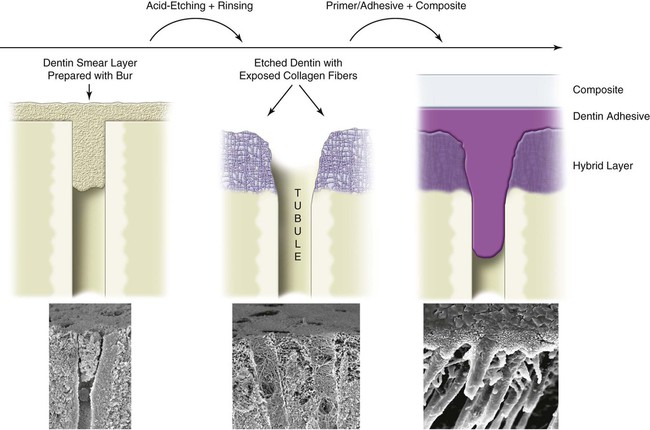
The classic concepts of operative dentistry were challenged in the 1980s and 1990s by the introduction of new adhesive techniques, first for enamel and then for dentin. Nevertheless, adhesion to dentin remains difficult. Adhesive materials can interact with dentin in different ways—mechanically, chemically, or both.7,9,30–33 The importance of micromechanical bonding, similar to what occurs in enamel bonding, has become accepted.30,34,35 Dentin adhesion relies primarily on the penetration of adhesive monomers into the network of collagen fibers left exposed by acid etching (Fig. 4-7).35,36 However, for adhesive materials that do not require etching, such as glass ionomer cements and some phosphate-based self-etch adhesives, chemical bonding between polycarboxylic or phosphate monomers and hydroxyapatite has been shown to be an important part of the bonding mechanism.32,37,38 Contemporary strategies for bonding to dentin are summarized in Table 4-1.
Table 4-1
Current Strategies for Adhesion of Resins to Dentin
| Etchant (E) | Primer (P) | Bonding Agent (B) | |
| Three-step etch-and-rinse* E + P + B |
etch-and-rinse
E + [PB]
Penetrates into the dentin tubules to form resin tags
The first coat applied on etched dentin works as a primer—it increases the surface free energy of dentin
The second coat (and third, fourth, and so on) acts as the bonding agent used in three-step systems—it fills the spaces between the dense network of collagen fibers
self-etch
[EP] + B
Enamel etch is typically shallow
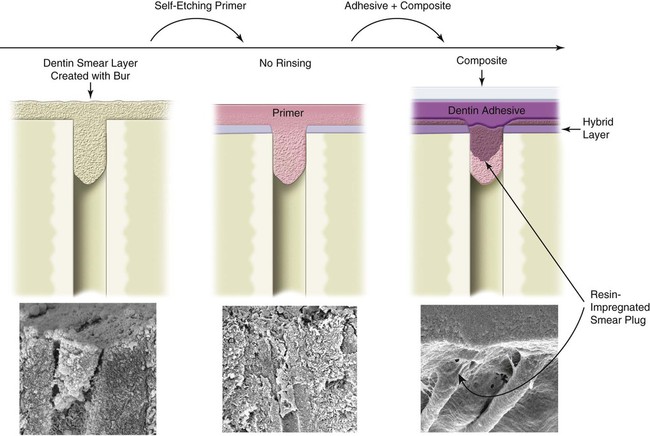
The self-etching primer (SEP) does not remove the smear layer, but fixes it and exposes about 0.5–1 µm of intertubular collagen because of its acidity (pH = 1.2–2.0)
The smear plug is impregnated with acidic monomers, but it is not removed; some SEP monomers bond chemically to dentin
When it impregnates the smear plug, the SEP prepares the pathway for the penetration of the subsequently placed fluid resin into the microchannels that permeate the smear plug
self-etch
[EPB]
Etches enamel, but etch pattern is typically shallow
Incorporates the smear layer into interface
Being an aqueous solution of a phosphonated monomer, it demineralizes and penetrates dentin simultaneously, leaving a precipitate on the hybrid layer
Forms a thin layer of adhesive, leading to low bond strengths; a multi-coat approach is recommended; an extra layer of a hydrophobic bonding resin improves bond strengths and clinical performance
Incompatible with self-cure composite resins unless coated with an hydrophobic bonding resin
*Although the meaning of the two terms is the same, the term “etch-and-rinse” is preferred over “total-etch.”
Challenges in Dentin Bonding
Substrate
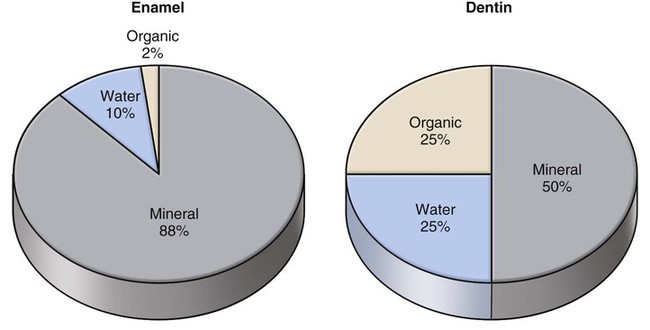
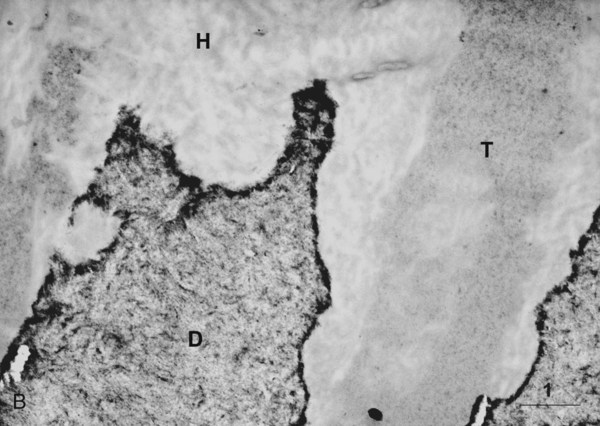
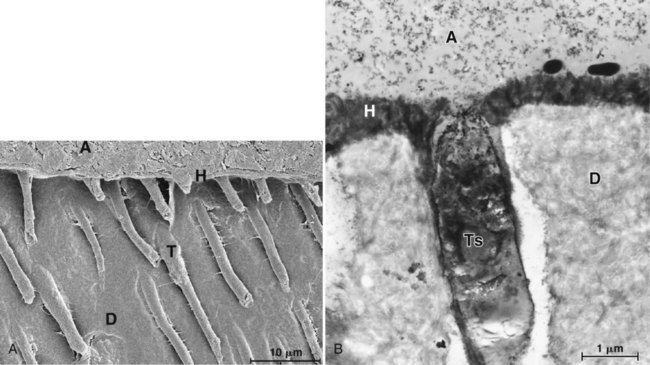
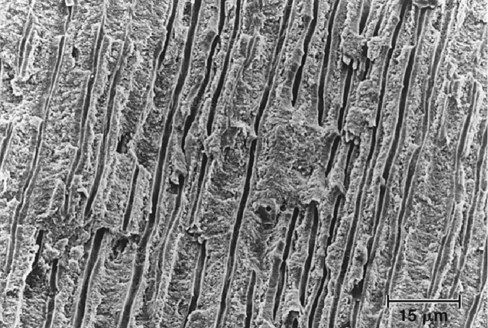
Bonding to enamel is a relatively simple process, without major technical requirements or difficulties. Bonding to dentin presents a much greater challenge. Several factors account for this difference between enamel and dentin bonding. Enamel is a highly mineralized tissue composed of more than 90% (by volume) hydroxyapatite, whereas dentin contains a substantial proportion of water and organic material, primarily type I collagen (Fig. 4-8). Dentin also contains a dense network of tubules that connect the pulp with the dentinoenamel junction (DEJ) (Fig. 4-9). A cuff of hypermineralized dentin called peritubular dentin lines the tubules. The less mineralized intertubular dentin contains collagen fibrils with the characteristic collagen banding (Fig. 4-10). Intertubular dentin is penetrated by submicron channels, which allow the passage of tubular liquid and fibers between neighboring tubules, forming intertubular anastomoses.
Dentin is an intrinsically hydrated tissue, penetrated by a maze of fluid-filled tubules. Movement of fluid from the pulp to the DEJ is a result of a slight but constant pulpal pressure.39 Pulpal pressure has a magnitude of 25-30 mm Hg or 34 to 40 cm H2O.40,41
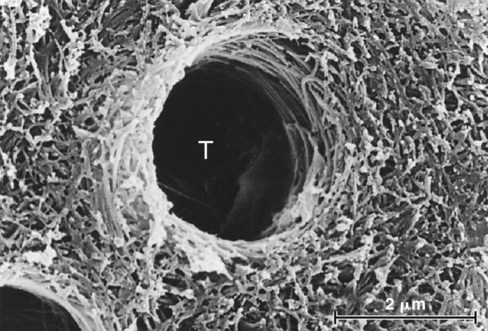
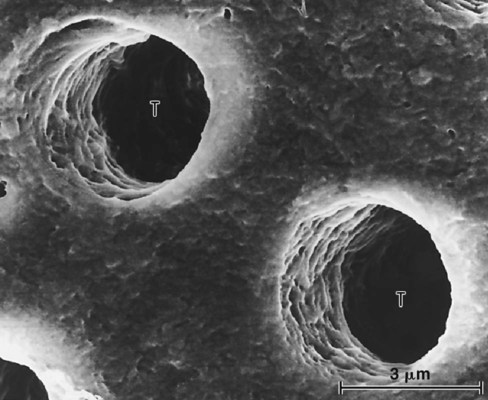
Dentinal tubules enclose cellular extensions from the odontoblasts and are in direct communication with the pulp (Fig. 4-11). Inside the tubule lumen, other fibrous organic structures such as the lamina limitans are present, which substantially decreases the functional radius of the tubule. The relative area occupied by dentin tubules decreases with increasing distance from the pulp. The number of tubules decreases from about 45,000/mm2 close to the pulp to about 20,000/mm2 near the DEJ.42 The tubules occupy an area of only 1% of the total surface near the DEJ, whereas they occupy 22% of the surface close to the pulp.43 The average tubule diameter ranges from 0.63 µm at the periphery to 2.37 µm near the pulp.44
Adhesion can be affected by the remaining dentin thickness after tooth preparation. Bond strengths are generally less in deep dentin than in superficial dentin.45–47 Nevertheless, some dentin adhesives, including one-step self-etch adhesives, do not seem to be affected by dentin depth.48
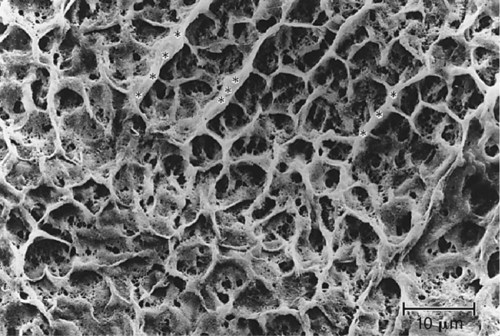
Whenever tooth structure is prepared with a bur or other instrument, residual organic and inorganic components form a “smear layer” of debris on the surface.49,50 The smear layer fills the orifices of dentin tubules, forming “smear plugs” (Fig. 4-12), and decreases dentin permeability by nearly 90%.51 The composition of the smear layer is basically hydroxyapatite and altered denatured collagen. This altered collagen can acquire a gelatinized consistency because of the friction and heat created by the preparation procedure.52 Submicron porosity of the smear layer still allows for diffusion of dentinal fluid.53 Removal of the smear layer and smear plugs with acidic solutions results in an increase of the fluid flow onto the exposed dentin surface. This fluid can interfere with adhesion because hydrophobic resins do not adhere to hydrophilic substrates, even if resin tags are formed in the dentin tubules.49,54
Several additional factors affect dentin permeability. Besides the use of vasoconstrictors in local anesthetics, which decrease pulpal pressure and fluid flow in the tubules, factors such as the radius and length of the tubules, the viscosity of dentin fluid, the pressure gradient, the molecular size of the substances dissolved in the tubular fluid, and the rate of removal of substances by the blood vessels in the pulp affect permeability. All of these variables make dentin a dynamic substrate and consequently a difficult substrate for bonding.43,55
Stresses at the Resin–Dentin Interface
Composites shrink as they polymerize, creating considerable stresses within the composite mass, depending on the configuration of the preparation.56–59 When the composite is bonded to one surface only (e.g., for a direct facial veneer), stresses within the composite are relieved by flow from the unbonded surface. Stress relief within a three-dimensional bonded restoration is limited, however, by its configuration factor (C-factor).60 In an occlusal preparation, composite is bonded to five tooth surfaces—mesial, distal, buccal, lingual, and pulpal. The occlusal surface of the composite is the only “free” or unrestrained surface. In such a situation, the ratio between the number of bonded surfaces and the number of unbonded surfaces is 5 : 1, giving the restoration a configuration factor = 5. Stress relief is limited because flow can occur only from the single free surface.60,61
Unrelieved stresses in the composite contribute to internal bond disruption and marginal gaps around restorations that increase microleakage and potential postoperative sensitivity.62 The C-factor might be partially responsible for the decrease in bond strengths observed when deep dentin is bonded as part of a three-dimensional preparation.63
It has been reported that immediate bond strengths of approximately 17 MPa are necessary to resist the contraction stresses that develop in the composite during polymerization, to prevent marginal debonding.58,64 Water absorption by the resin might compensate for the effect of the polymerization shrinkage, as the resin might expand and seal off marginal gaps, but this occurs only over a relatively long time.65 Water absorption is directly proportional to the resin content.66
Enamel bond strengths usually are sufficient to prevent the formation of marginal gaps by polymerization contraction stresses. These stresses might, however, be powerful enough to cause enamel defects at the margins.67 Extension of the enamel cavosurface bevel helps improve the enamel peripheral seal.56,68
Each time a restoration is exposed to wide temperature variations in the oral environment (e.g., drinking coffee and eating ice cream), the restoration undergoes volumetric changes of different magnitude compared with those of the tooth structure. This occurs because the linear coefficient of thermal expansion of the composite is about four times greater than that of the tooth structure. Microleakage around dentin margins is potentiated by this discrepancy in linear coefficient of thermal expansion between the restoration and the substrate.69
Loading and unloading of restored teeth can result in transitional or permanent interfacial gaps.70 Additionally, the tooth substrate itself might be weakened by cyclic loading.71 A study found that 71% of Class V composite restorations in third molars with antagonists have significantly more leakage than restorations placed in teeth without opposing contact.72 Another study found that cyclic loading and preparation configuration significantly reduced the bond strengths of self-etch and etch-and-rinse adhesives.73,74
Development
Beginning
During the 1950s, it was reported that a resin containing glycerophosphoric acid dimethacrylate (GPDM) could bond to a hydrochloric acid–etched dentin surface.75 (Note: A complete listing of the chemical names mentioned in this chapter is provided in Table 4-2.) The bond strengths of this primitive adhesion technique were severely reduced by immersion in water. A few years before that report, another researcher had used the same monomer chemically activated with sulfinic acid, and that combination would later be known commercially as Sevriton Cavity Seal (Amalgamated Dental Company, London, England).76,77
Table 4-2
Abbreviations Commonly Used in Dentin/Enamel Adhesion Literature and in This Chapter
| Abbreviation | Chemical Name |
| Bis-GMA | Bisphenol-glycidyl methacrylate |
| EDTA | Ethylenediamine tetra-acetic acid |
| GPDM | Glycerophosphoric acid dimethacrylate |
| HEMA | 2-Hydroxyethyl methacrylate |
| 10-MDP | 10-Methacryloyloxy decyl dihydrogen phosphate |
| 4-META | 4-Methacryloxyethyl trimellitate anhydride |
| MMEP | Mono (2-methacryloxy) ethyl phthalate |
| NPG-GMA | N-phenylglycine glycidyl methacrylate |
| PENTA | Dipentaerythritol penta-acrylate monophosphate |
| Phenyl-P | 2-(Methacryloxy) ethyl phenyl hydrogen phosphate |
First Generation
The development of the surface-active co-monomer NPG-GMA was the basis for Cervident (S.S. White Burs, Inc., Lakewood, NJ), which is considered the first-generation dentin bonding system.31,78 Theoretically, this comonomer could chelate with calcium on the tooth surface to generate water-resistant chemical bonds of resin to dentinal calcium.79,80 The in vitro dentin bond strengths of this material were, however, in the range of only 2 to 3 MPa.81 Likewise, the in vivo results were discouraging; Cervident had poor clinical results when used to restore non-carious cervical lesions without mechanical retention.82
Second Generation
In 1978, the Clearfil Bond System Fc was introduced in Japan (Kuraray Co., Ltd., Osaka, Japan). Generally recognized as the first product of the second-generation of dentin adhesives, it was a phosphate-ester material (phenyl-P and hydroxyethyl methacrylate [HEMA] in ethanol). Its mechanism of action was based on the polar interaction between negatively charged phosphate groups in the resin and positively charged calcium ions in the smear layer.81 The smear layer was the weakest link in the system because of its relatively loose attachment to the dentin surface. Examination of both sides of failed bonds revealed the presence of smear layer debris.83
Several other phosphate-ester dentin bonding systems were introduced in the early 1980s, including Scotchbond (3M EPSE Dental Products, St. Paul, MN), Bondlite (Kerr Corporation, Orange, CA), and Prisma Universal Bond (DENTSPLY Caulk, Milford, DE). These second-generation dentin bonding systems typically had in vitro bond strengths of only 1 to 5 MPa, which was considerably below the 10 MPa value estimated as the threshold value for acceptable in vivo retention.9,52 In addition to the problems caused by the loosely attached smear layer, these resins were relatively devoid of hydrophilic groups and had large contact angles on intrinsically moist surfaces.84 They did not wet dentin well, did not penetrate the entire depth of the smear layer, and, therefore, could not reach the superficial dentin to establish ionic bonding or resin extensions into the dentinal tubules.52 Whatever bonding did occur was due to interaction with calcium ions in the smear layer.85
The in vitro performance of second-generation adhesives after 6 months was unacceptable.86 The bonding material tended to peel from the dentin surface after water storage, indicating that the interface between dentin and some types of chlorophosphate ester–based materials was unstable.86,87 The in vivo performance of these materials was found to be clinically unacceptable 2 years after placement in cervical tooth preparations without additional retention, such as beveling and acid-etching.88,89
Third Generation
The concept of phosphoric acid-etching of dentin before application of a phosphate ester-type bonding agent was introduced by Fusayama et al in 1979.90 Because of the hydrophobic nature of the bonding resin, however, acid-etching did not produce a significant improvement in dentin bond strengths, despite the flow of the resin into the open dentinal tubules.54,91 Pulpal inflammatory responses were thought to be triggered by the application of acid on dentin surfaces, providing another reason to avoid etching.92,93 Nevertheless, continuing the etched dentin philosophy, Kuraray introduced Clearfil New Bond in 1984. This phosphate-based material contained HEMA and a 10-carbon molecule known as 10-MDP, which includes long hydrophobic and short hydrophilic components.79
Most other third-generation materials were designed not to remove the entire smear layer but, rather, to modify it and allow penetration of acidic monomers, such as phenyl-P or PENTA. Despite promising laboratory results, some of the bonding mechanisms never resulted in satisfactory clinical results.89,94
Treatment of the smear layer with acidic primers was proposed using an aqueous solution of 2.5% maleic acid, 55% HEMA, and a trace of methacrylic acid (Scotchbond 2, 3M ESPE Dental Products).79 Scotchbond 2 was the first dentin bonding system to receive “provisional” and “full acceptance” from the American Dental Association (ADA).95 With this type of smear layer treatment, manufacturers effectively combined the dentin etching philosophy advocated in Japan with the more cautious approach advocated in Europe and the United States. The result was preservation of a modified smear layer with slight demineralization of the underlying intertubular dentin surface. Clinical results were mixed, with some reports of good performance and some reports of poor performance.88,89
The removal of the smear layer using chelating agents such as EDTA was recommended in the original Gluma system (Bayer Dental, Leverkusen, Germany) before the application of a primer solution of 5% glutaraldehyde and 35% HEMA in water. The effectiveness of this system might have been impaired, however, by the manufacturer’s questionable recommendation of placing the composite over uncured unfilled resin.89
Current Options for Resin–Dentin Bonding
Three-Step Etch-and-Rinse Adhesives
Although the smear layer acts as a “diffusion barrier” that reduces the permeability of dentin, it also can be considered an obstacle that must be removed to permit resin bonding to the underlying dentin substrate.51 Based on that consideration, a fourth generation of dentin adhesives was introduced for use on acid-etched dentin.96 Removal of the smear layer via acid-etching led to significant improvements in the in vitro bond strengths of resins to dentin.97–100 Because the clinical technique involves simultaneous application of an acid to enamel and dentin, this method was originally known as the “total-etch” technique. Now more commonly called etch-and-rinse technique, it was the most popular strategy for dentin bonding during the 1990s and remains somewhat popular today (Fig. 4-13).
Application of acid to dentin results in partial or total removal of the smear layer and demineralization of the underlying dentin.90 Acids demineralize intertubular and peri-tubular dentin, open the dentin tubules, and expose a dense filigree of collagen fibers (see Fig. 4-7), increasing the microporosity of the intertubular dentin (Fig. 4-14).35,101 Dentin is demineralized by up to approximately 7.5 µm, depending on the type of acid, application time, and concentration.35,101
Despite the obvious penetration of early adhesives into the dentinal tubules, etching did not result in a significant improvement in bond strengths, possibly as a result of the hydrophobic nature of the phosphonated resin.91 On the basis of concerns about the potential for inflammatory pulpal responses, acids were believed to be contraindicated for direct application on dentin, and the total-etch technique was not readily accepted in Europe or the United States. Adhesive systems based on the total-etch philosophy have proved successful, however, in vitro and in vivo.89,102–104 Laboratory shear bond strengths usually vary from 17 to 30 MPa, which are similar to the values typically obtained on enamel.
The acid-etching step not only alters the mineral content of the dentin substrate but also changes its surface free energy.33,96 The latter is an undesirable effect because for good interfacial contact, any adhesive must have a low surface tension, and the substrate must have a high surface free energy.34,52,87 Substrates are characterized as having low or high surface energy. Among dental materials, hydroxyapatite and glass ionomer cement filler particles are high-energy substrates, whereas collagen and composite have low-energy surfaces.2 Consequently, dentin consists of two distinct substrates, one of high surface energy (hydroxyapatite) and one of low surface energy (collagen). After etching, the dense web of exposed collagen is a low surface energy substrate.86 A correlation exists between the ability of an adhesive to spread on the dentin surface and the concentration of calcium on that same surface.105 The primer in a three-step system is designed to increase the critical surface tension of dentin, and a direct correlation between surface energy of dentin and shear bond strengths has been shown.46
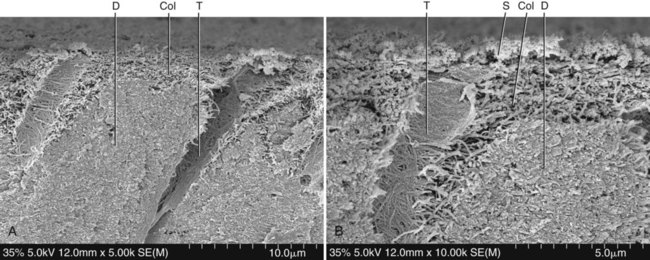
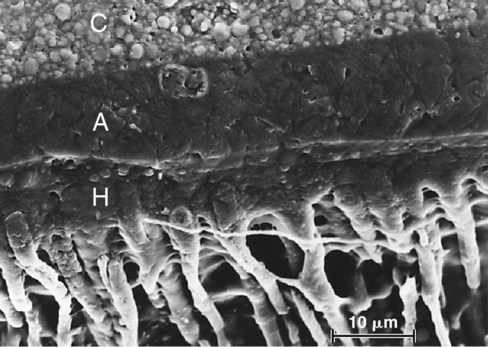
When primer and bonding resin are applied to etched dentin, they penetrate the intertubular dentin, forming a resin–dentin interdiffusion zone, or hybrid layer. They also penetrate and polymerize in the open dentinal tubules, forming resin tags. For most etch-and-rinse adhesives, the ultramorphologic characterization of the transition between the hybrid layer and the unaffected dentin suggests that an abrupt shift from hybrid tissue to mineralized tissue occurs, without any empty space or pathway that could result in leakage (Figs. 4-15 and 4-16). The demarcation line seems to consist of hydroxyapatite crystals embedded in the resin from the hybrid layer (see Fig. 4-16, B). For self-etch systems, the transition is more gradual, with a superficial zone of resin-impregnated smear residues and a deeper zone, close to the unaffected dentin, rich in hydroxyapatite crystals (Fig. 4-17).
Two-Step Etch-and-Rinse Adhesives
In vitro dentin bond strengths have improved so much that they approach the level of enamel bonding.27 Therefore, much of the research and development (R&D) has focused on the simplification of the bonding procedure. A number of dental materials manufacturers are marketing a simplified, two-step etch-and-rinse adhesive system. Some authors refer to these as fifth-generation adhesives, and they are sometimes called “one-bottle” systems because they combine the primer and bonding agent into a single solution. A separate etching step still is required.
Two-Step Self-Etch Systems
An alternative bonding strategy is the self-etch approach (Figs. 4-17 and 4-18). Some self-etch systems are most accurately described as nonrinsing conditioners or self-priming etchants. Examples include NRC Non-Rinse Conditioner (DENTSPLY DeTrey, Konstanz, Germany) and Tyrian SPE (Bisco, Inc.). NRC and Tyrian SPE required the subsequent application of a separate adhesive, the same used with the etch-and-rinse technique (Prime & Bond NT [DENTSPLY Caulk] with NRC, and One-Step Plus [Bisco, Inc.] with Tyrian SPE). Nonrinsing conditioners did not etch enamel to the same depth as phosphoric acid, and did not provide higher bond strengths or better clinical performance than phosphoric acid etchants.106,107
Another type of acidic conditioner was introduced in Japan—the self-etching primers (SEPs)—and has proved to be more successful. These acidic primers include a phosphonated resin molecule that performs two functions simultaneously—etching and priming of dentin and enamel. In contrast to conventional etchants, SEPs are not rinsed off. The bonding mechanism of SEPs is based on the simultaneous etching and priming of enamel and dentin, forming a continuum in the substrate and incorporating smear plugs into the resin tags (Fig. 4-19).108,109 In addition to simplifying the bonding technique, the elimination of rinsing and drying steps reduces the possibility of over-wetting or over-drying, either of which can affect adhesion adversely.98,99 Also, water is always a component of SEPs because it is needed for the acidic monomers to ionize and trigger demineralization of hard dental tissues; this makes SEPs less susceptible to variations in the degree of substrate moisture but more susceptible to chemical instability due to hydrolytic degradation.110–112
One disadvantage of SEPs that are currently available is that they do not etch enamel as well as phosphoric acid, particularly if the enamel has not been instrumented. The seal of enamel margins in vivo might be compromised.113,114 When enamel bonds are stressed in the laboratory by thermal cycling, SEPs are more likely than etch-and-rinse systems to undergo deterioration.115 This decrease in bond strengths with thermal fatigue might be a sign that a potential exists for enamel microleakage when SEPs are employed to bond to enamel. In a 10-year recall of an older generation SEP, 39 of 44 restorations had marginal discoloration.116 The enamel bond strengths of some newer SEPs approach the enamel bond strengths of phosphoric acid–based adhesives, however, suggesting that SEPs are gradually being developed to replace etch-and-rinse adhesive systems.
Because they are user-friendly and do not require the etching and rinsing step, SEPs such as Clearfil SE Bond (Kuraray) have become very popular.117 Clearfil SE Bond contains an aqueous mixture of a phosphoric acid ester monomer (10-MDP), with a much higher pH than that of phosphoric acid etchants.118 Although the pH of a 34% to 37% phosphoric acid gel is much lower than 1.0, the pH of Clearfil SE Primer (Kuraray) is 1.9 to 2.0.101,106 SEPs have been classified in three categories: mild, moderate, and aggressive, with Clearfil SE Bond being a mild SEP.106 Mild SEPs tend to provide excellent dentin bond strengths and poorer enamel bonds, whereas more aggressive self-etch systems provide the reverse. Clearfil SE Bond resulted in 98% retention rate in Class V composite restorations at 8 years with or without separate enamel etching of the margins, which did improve marginal adaptation.119 In posterior restorations, Clearfil SE Bond resulted in 100% retention rate at 2 years with a tendency for deterioration of the composite margins compared with the etch-and-rinse control Single Bond.102 The clinical success of Clearfil SE Bond might be a result of its chemical composition, specifically the monomer 10-MDP. This monomer bonds chemically to hydroxyapatite by forming stable calcium-phosphate salts without causing strong decalcification. The chemical bonding formed by 10-MDP is more stable in water than that of other monomers used in the composition of self-etch adhesives, such as 4-META and phenyl-P.120
SEPs are less technique sensitive than are etch-and-rinse adhesives. Additionally, SEPs are less likely to result in a discrepancy between the depth of demineralization and the depth of resin infiltration because SEPs demineralize and infiltrate dentin simultaneously.118 SEPs do not remove the smear layer from dentin completely (see Figs. 4-17 and 4-18), which is the main reason that they might result in less postoperative sensitivity compared with etch-and-rinse adhesives.55,121
Despite the prevailing opinion that SEPs cause less postoperative sensitivity compared with etch-and-rinse systems, the few clinical studies comparing these in posterior restorations have reported mixed results.55,121 Nevertheless, recent clinical studies have shown no relationship between the type of adhesive and the occurrence of postoperative sensitivity.123–129 One clinical study found no differences in postoperative sensitivity from 2 weeks to 6 months between an etch-and-rinse adhesive (Prime & Bond NT) and an SEP (Clearfil SE Bond) used in Class I and Class II composite restorations. These results suggest that the restorative technique is more important than the material itself.
One-Step Self-Etch Adhesives
Continuing the trend toward simplification, no-rinse, self-etching materials that incorporate the fundamental steps of etching, priming, and bonding into one solution have become increasingly popular. In contrast to conventional adhesive systems that contain an intermediate light-cured, low-viscosity bonding resin to join the composite restorative material to the primed dentin–enamel substrate, these one-step self-etch or “all-in-one” adhesives contain uncured ionic monomers that contact the composite restorative material directly.128,129 Their acidic unreacted monomers are responsible, in part, for the incompatibility between these all-in-one adhesives and self-cured composites (discussed later).129 Additionally, one-step adhesives tend to behave as semi-permeable membranes, resulting in a hydrolytic degradation of the resin–dentin interface.110 Because these adhesives must be acidic enough to be able to demineralize enamel and penetrate dentin smear layers, the hydrophilicity of their resin monomers, usually organophosphates and carboxylates, also is high. Some of these resin monomers are too hydrophilic, which makes them liable to water degradation.111,130
Many one-step self-etch adhesives with etching, priming, and bonding functions delivered in a single solution are now available, including AdheSE One F (Ivoclar Vivadent), Adper Easy Bond (3M ESPE), All-Bond SE (Bisco Inc.), Bond Force (Tokuyama Dental, Tokyo, Japan), Clearfil S3 Bond (Kuraray), iBOND Self-Etch (Heraeus Kulzer, South Bend, IN), OptiBond All-in-One (Kerr Corporation), and Xeno V+ (DENTSPLY DeTrey). As with the SEP systems, the pH of an all-in-one, self-etching adhesive affects its clinical properties. Also, application of multiple coats, such as four consecutive coats for Xeno III (DENTSPLY DeTrey) or five consecutive coats for iBond (Heraeus Kulzer), significantly increases dentin bond strengths and decreases leakage, suggesting that some of the “all-in-one” adhesives might not coat the dentin surface uniformly.131
A clinical study of Adper Prompt L-Pop (3M EPSE) reported a 35% failure rate at 1 year in Class V restorations, although the material used in this study was an earlier version.132 A modified version of this material, Adper Prompt, had significantly worse marginal adaptation than Scotchbond Multi-Purpose in noncarious cervical lesions at 2 years.133 Similar findings were reported for Adper Prompt L-Pop in another Class V clinical study. Although Adper Prompt L-Pop resulted in similar retention rates as Adper Single Bond at 3 years, the self-etch adhesive resulted in significantly higher incidence of marginal discoloration.134
For iBond, marginal discoloration and marginal adaptation were much less than ideal at 3 years.135 Another clinical trial in noncarious Class V lesions compared different generations of dentin adhesives—three-step etch-and-rinse, two-step etch-and-rinse, two-step self-etch, and one-step (or all-in-one) self-etch.136 Out of four different adhesives from the same manufacturer, only the three-step etch-and-rinse adhesive resulted in retention rate greater than 90% at 18 months necessary to fulfill the ADA requirement for full acceptance.96 In a clinical study with posterior composite restorations, iBond resulted in a significant decrease in the quality of color match, marginal staining, and marginal adaptation at 2 years.137
The in vitro and clinical behavior of all-in-one (one-step) self-etch adhesives improves when the clinician adds an extra coat of a hydrophobic bonding layer.138–140 In a recent clinical study in Class V lesions, the one-step self-etch adhesive Clearfil S3 Bond resulted in 77.3% retention rate at 18 months.139 For the group to which an extra layer of a thick bonding resin was added (Scotchbond Multi-Purpose Adhesive), the retention rate increased to 93.4% at 18 months. In the same study, iBond, also a one-step self-etch adhesive, resulted in a 60% retention rate at 18 months. However, the retention increased to 83% when a coat of the same hydrophobic resin was applied over the cured iBond, transforming it in a two-step system. This behavior of one-bottle self-etch adhesives may be related to their behavior as semi-permeable membranes in vitro and in vivo.110,141 Simplified self-etch adhesives do not provide a hermetic seal for vital deep dentin as demonstrated by transudation of dentinal fluid across the polymerized adhesives to form fluid droplets on the surface of the adhesive.111
Moist versus Dry Dentin Surfaces with Etch-and-Rinse Adhesives
Because vital dentin is inherently wet, complete drying of dentin is difficult to achieve clinically.99,142 Water has been considered an obstacle for attaining an effective adhesion of resins to dentin, so research has shifted toward the development of dentin adhesives that are compatible with humid environments. Many adhesives combine hydrophilic and hydrophobic monomers in the same bottle, dissolved in an organic solvent such as ethanol or acetone. The “moist bonding” technique used with etch-and-rinse adhesives prevents the spatial alterations (i.e., collagen collapse) that occur on drying demineralized dentin (Fig. 4-20; compare with Fig. 4-14).99 Such alterations might prevent the monomers from penetrating the labyrinth of nanochannels formed by dissolution of hydroxyapatite crystals between collagen fibers.143,144
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses