Direct Esthetic Restorative Materials
Objectives
After reading this chapter, the student should be able to:
Composites
1. Describe the uses of all-purpose and posterior composites.
< ?mpslid E1?>< ?mpslid S2?>
2. Indicate components used in composites.
< ?mpslid E2?>< ?mpslid S3?>
3. Describe properties of composites, and indicate their clinical importance.
< ?mpslid E3?>< ?mpslid S4?>
4. Describe the manipulation of composites.
< ?mpslid E4?>
Composites for Special Applications
< ?mpslid E5?>< ?mpslid S6?>
2. Indicate components used in composites for special applications.
< ?mpslid E6?>< ?mpslid S7?>
3. Describe properties of composites for special applications.
< ?mpslid E7?>< ?mpslid S8?>
4. Describe the manipulation of composites for special applications.
< ?mpslid E8?>
Compomers
1. Describe the uses of compomers.
< ?mpslid E9?>< ?mpslid S10?>
2. Indicate components used in compomers.
< ?mpslid E10?>< ?mpslid S11?>
3. Describe properties of compomers.
< ?mpslid E11?>< ?mpslid S12?>
4. Describe the manipulation of compomers.
< ?mpslid E12?>
Glass Ionomers
1. Describe the uses of glass ionomers.
< ?mpslid E13?>< ?mpslid S14?>
2. Indicate components used in glass ionomers.
< ?mpslid E14?>< ?mpslid S15?>
3. Describe properties of glass ionomers.
< ?mpslid E15?>< ?mpslid S16?>
4. Describe the manipulation of glass ionomers.
< ?mpslid E16?>
Resin-Modified Glass Ionomers
1. Describe the uses of resin-modified glass ionomers.
< ?mpslid E17?>< ?mpslid S18?>
2. Indicate components used in resin-modified glass ionomers.
< ?mpslid E18?>< ?mpslid S19?>
3. Describe properties of resin-modified glass ionomers.
< ?mpslid E19?>< ?mpslid S20?>
4. Describe the manipulation of resin-modified glass ionomers.
< ?mpslid E20?>
Bonding Agents
1. Indicate components used in bonding agents.
< ?mpslid E21?>< ?mpslid S22?>
2. Describe properties of bonding agents, and indicate their clinical importance.
< ?mpslid E22?>< ?mpslid S23?>
3. Describe the manipulation of bonding agents.
< ?mpslid E23?>< ?mpslid S24?>
4. List dental materials that can interfere with the polymerization of bonding agents.
< ?mpslid E24?>
Light-Curing Units
1. List desirable features of light-curing units.
< ?mpslid E25?>< ?mpslid S26?>
2. Describe precautions for protecting eyes of patients and staff.
< ?mpslid E26?>< ?mpslid S27?>
3. Describe four factors that influence exposure times for polymerization of composites.
< ?mpslid E27?>
Key Terms
Abfraction
Catalyst
Compomer
Compules
Copolymer
Liner
Nanofiller
Oligomer
Opaque
Primer/adhesive
Silane coupling agent
The need for restorative materials that have the appearance of natural tooth tissue and that can be placed directly into a cavity preparation in a paste condition is great. The patient desires esthetic restorations, particularly in the anterior portion of the mouth, and a direct filling material is advantageous in terms of the time required and the cost of the restoration. Selection of a material is made based on a need for esthetics, fluoride release, wear resistance, strength, and ease of use.
Currently, four types of materials are being used as direct esthetic dental restorations: (1) composites, (2) compomers, (3) resin-modified glass ionomers, and (4) glass ionomers. Composites were introduced about 1960 and now dominate the materials used for direct esthetic restorations. Glass ionomers were introduced in 1972 and have been used primarily for restoration of cervically eroded areas. Resin-modified glass ionomers were introduced in the early 1990s to provide better esthetics than glass ionomers. Compomers were introduced in 1995 to provide improved handling and fluoride release compared with composites.
Composites are esthetically pleasing, strong, and wear resistant but have low or no fluoride release. Compomers are less wear resistant but are esthetically pleasing and release fluoride. Resin-modified glass ionomers release more fluoride than compomers do but are not as wear resistant and are not used in posterior restorations. Glass ionomers release the most fluoride and are best for patients with a high risk of caries in low-stress applications. Uses of composites, compomers, resin-modified ionomers, and glass ionomers are listed in Table 4-1. Typical products, including composites for special applications, are listed in Table 4-2.
TABLE 4-1
Uses of Composites, Compomers, Resin-Modified Glass Ionomers, and Glass Ionomers
| TYPE | USES |
| All-purpose composite | Class I, II, III, IV, V, patients with low risk of caries |
| Microfilled composite | Class III, V |
| Nanofilled composite | Class I, II, III, IV, V |
| Packable composite | Class I, II, VI (mesial, occlusal, distal = MOD) |
| Flowable composite | Cervical lesions, pediatric restorations, small, low-stress-bearing restorations |
| Laboratory composite | Class II, three-unit bridge (with fiber reinforcement) |
| Compomer | Cervical lesions, Class III primary teeth, Class I, II restorations in children, Class II (with sandwich technique), patients with medium risk of caries |
| Resin-modified glass ionomer | Cervical lesions, Class III, V, II (with sandwich technique), pediatric restorations primary teeth, Class I restorations in children, sandwich technique (Class II), patients with a high risk of caries |
| Glass ionomer | Cervical lesions, Class V restorations in adults in whom esthetics are less important than that of other types, patients with a high risk of caries |
TABLE 4-2
< ?comst?>
| TYPE | PRODUCT | MANUFACTURER |
| Microhybrid composites | Estelite Sigma Quick | Tokuyama Dental America (Burlingame, CA) |
| Gradia Direct | GC America (Alsip, IL) | |
| Microfilled composites | Durafill VS | Heraeus (South Bend, IN) |
| Nanofilled composites | Filtek Supreme Plus | 3M ESPE (St. Paul, MN) |
| Universal Restorative | ||
| Simile | Pentron Clinical (Wallingford, CT) | |
| Tetric EvoCeram | Ivoclar Vivadent (Amherst, NY) | |
| Packable composites | Alert | Pentron Clinical |
| Surefil | DENTSPLY Caulk (Milford, DE) | |
| Flowable composites | Filtek Supreme Plus | 3M ESPE |
| Flowable Restorative | ||
| Palfique Estelite LV | Tokuyama Dental America | |
| Surefil SDR flow | DENTSPLY Caulk | |
| Tetric EvoFlow | Ivoclar Vivadent | |
| Flowable composites (self-adhesive) | Fusion Liquid Dentin | Pentron Clinical |
| Laboratory composites | Tescera ATL | Bisco Dental Products (Schaumburg, IL) |
| Compomers | Compoglass F | Ivoclar Vivadent |
| Dyract eXtra | DENTSPLY Caulk | |
| Glass ionomers | GC Fuji II | GC America |
| Resin-modified glass ionomers | GC Fuji II LC | GC America |
| Ketac Nano | 3M ESPE | |
| Composites for special applications | ||
| Core buildup | LuxaCore Z-Dual | DMG America (Englewood, NJ) |
| Clearfil PhotoCore PLT | Kuraray America (New York, NY) | |
| Provisional composites | Luxatemp Fluorescence | DMG America |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Composites
Composite restoratives generally are recommended for Classes III to V and for Class I when occlusal stress is not a problem and appearance is crucial. Although less durable than amalgam, composites designed for Class II posterior applications are used in about 50% of these restorations. Composites also can be classified as all-purpose, packable, flowable, laboratory, microfilled, and nanofilled composites, uses of which are listed in Table 4-1. Typical products are listed in Table 4-2. Composites are also used for provisional restorations and core buildups and in fiber-reinforced posts.
Composition and Reaction
Composites consist of three phases: resin matrix, dispersed inorganic filler particles, and silane coupling agent on the filler particles to produce a good bond between the matrix and the filler.
Filler Size
Currently, most composites have fillers with average diameters of 0.2 to 3 µm (fine particles) or 0.04 µm (microfine particles). The fraction of particles having diameters of 0.04 µm varies from a few percent to 35% by weight. The volume percentage of filler particles is lower than the weight percentage because of the higher density of the filler compared with that of the polymer matrix. Nanofilled composites have fillers ranging in size from 1 to 10 nanometers (nm), although these fillers may be present as clusters of a larger size.
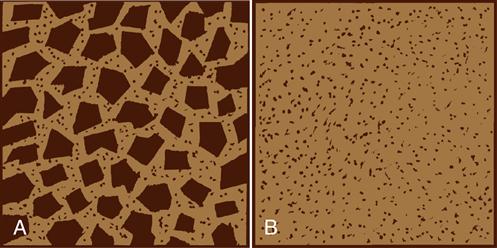
Figure 4-1 illustrates the two main classes of composites. Microhybrid composites (Figure 4-1, A) contain blends of fine and microfine filler particles with as much as 84% filler by weight. The microfine filler particles fit in spaces between the fine filler particles, producing a total filler concentration of 70% by volume, which results in improved properties.
Microfilled composites (Figure 4-1, B) contain microfine fillers with high surface areas. Only 35% to 50% by volume of these particles can be used with the resin matrix and still produce a paste of acceptable viscosity. Some microfilled composites use fillers that are polymer particles reinforced with microfine particles, which are then mixed with the resin matrix; these reinforced filler particles may be as large as 10 to 20 µm. These products allow the incorporation of more microfilled fillers and yield a paste with reasonable viscosity.
Filler Composition
Quartz, lithium aluminum silicate, and barium, strontium, zinc, or ytterbium glasses have been used as fine fillers. Microfine fillers are colloidal silica particles. Fine fillers that contain barium, strontium, zinc, or ytterbium atoms are radiopaque with the radiopacity proportional to the volume fraction of the filler. Quartz (crystalline silica) and lithium aluminum silicate are not radiopaque. Manufacturers specify if a composite is radiopaque or not. Radiopaque composites are used to restore posterior teeth.
Coupling Agents
To provide a good bond between the inorganic fillers and the resin matrix, manufacturers treat the surface of the filler with silane, which has groups that react with the inorganic filler and other groups that react with the organic matrix.
Resin Matrix
The most common resins are based on dimethacrylate (Bis-GMA, bisphenol A-glycidyl methacrylate) or urethane dimethacrylate (UDMA) oligomers. A highly simplified formula in which R represents any of a large number of organic groups (e.g., phenyl-, methyl-, carboxyl-, hydroxyl-, and amide-) follows:
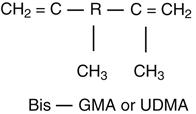
Bis-GMA and UDMA oligomers are viscous liquids to which low-molecular-weight monomers (dimethacrylates) are added to control the consistency of the composite paste. Oligomers and the low-molecular-weight monomers are characterized by carbon double bonds that react to convert them to a polymer.
Initiators and Accelerators
The principal system used to achieve polymerization (setting) is the visible light-curing system. In this system, the composite is polymerized by being exposed to an intense blue light. The light is absorbed by a diketone, which, in the presence of an organic amine, starts the polymerization reaction. Exposure times of 20 to 40 seconds are needed for polymerization. Because blue light is necessary to start the reaction, the diketone and amine can be in the same composite paste, and no reaction occurs until it is exposed to blue light. Light-curing units are described later in this chapter.
In self-curing systems, polymerization is accomplished with an organic peroxide initiator and an organic amine accelerator. The initiator and accelerator must be kept separated and not mixed until just before the restoration is placed.
Regardless of the system used, the following general reaction takes place:
< ?xml:namespace prefix = "mml" ns = "http://www.w3.org/1998/Math/MathML" />

Pigments
Inorganic pigments are added in small amounts so that the color of the composite matches the tooth structure. Typically, composites are provided in 10 or more shades, which cover the normal range of human teeth (yellow to gray). Highly pigmented tints can be mixed with the standard shades to match the color of teeth outside the normal range. Special shades for bleached teeth are also available.
Composites have been developed with enamel, dentin, cervical, and opaque shades for special techniques in esthetic dentistry. These multipurpose composites can be placed in one layer or several layers to improve esthetics. Examples of these special composites are listed in Table 4-2.
Properties
Important properties of composites include the following:
1. Low polymerization shrinkage
< ?mpslid E28?>< ?mpslid S29?>
< ?mpslid E29?>< ?mpslid S30?>
3. Coefficient of thermal expansion similar to tooth structure
< ?mpslid E30?>< ?mpslid S31?>
< ?mpslid E31?>< ?mpslid S32?>
< ?mpslid E32?>< ?mpslid S33?>
< ?mpslid E33?>< ?mpslid S34?>
7. High bond strength to enamel and dentin
< ?mpslid E34?>< ?mpslid S35?>
8. Good color match to tooth structure
< ?mpslid E35?>< ?mpslid S36?>
< ?mpslid E36?>< ?mpslid S37?>
10. Ease of finishing and polishing
< ?mpslid E37?>
Some of these qualities are more important for anterior or posterior applications. Values for a variety of properties are presented in Table 4-3 for microhybrid and microfilled composites. The nanofilled composites have values similar to those for the microhybrid composites.
TABLE 4-3
Properties of Microhybrid and Microfilled Composites
| PROPERTY | MICROHYBRID COMPOSITE∗ | MICROFILLED COMPOSITE |
| Polymerization shrinkage (% linear) | 1.0–1.7 | 2–3 |
| Thermal conductivity (10−4 cal/sec/cm2[°C/cm]) | 25–30 | 2–15 |
| Linear coefficient of thermal expansion (× 10−6/°C) | 25–38 | 55–68 |
| Water sorption (mg/cm2) | 0.3–0.6 | 1.2–2.2 |
| Radiopacity (mm Al)† | 2.7–5.7 | — |
| Compressive strength (MPa) | 200–340 | 230–290 |
| Diametral tensile strength (MPa) | 34–62 | 26–33 |
| Flexural strength (MPa) | 90–140 | — |
| Elastic modulus in compression (GPa) | 8–14 | 3–5 |
| Flexural modulus (GPa) | 5–18 | — |
| Knoop hardness (kg/mm2) | 55–80 | 22–36 |
| Bond strength to enamel and dentin with bonding agent (MPa) | 14–30 | 14–30 |
∗Nanofilled composites have properties similar to microhybrid composites.
†If advertised as radiopaque. Enamel is 4.0 mm Al (millimeters of aluminum) and dentin is 2.5 mm Al.
Polymerization Shrinkage
Microhybrid composites shrink less during setting than microfilled types because the microhybrid composites have less resin. Even with acid etching of enamel and dentin and use of bonding agents, stresses from polymerization shrinkage can exceed the bond strength of a composite to tooth structure, and, as a result, marginal leakage can occur.
Low-shrinkage and low-stress composites (GC Kalore, GC America, Alsip, Illinois; N’Durance, Septodont, Louisville, Colorado) have been introduced. These composites have modified resin and filler systems that result in reduced polymerization shrinkage and stress.
Two techniques have been proposed to overcome or minimize the effect of polymerization shrinkage. One method is to insert and polymerize the composite in layers, which reduces the effective shrinkage. The second method is to prepare a laboratory (indirect) composite inlay on a die and then to cement the inlay to the tooth with a thin layer of low-viscosity resin cement as discussed later in this chapter.
Thermal Conductivity
The thermal conductivity of a composite is much lower than that for metallic restorations (see Table 2-2) and closely matches that of enamel and dentin. Therefore, composites provide good thermal insulation for the dental pulp.
Thermal Expansion
Typical values for microhybrid and microfilled composites are shown in Table 4-3. Because the thermal expansion of composites is greater than that of tooth structure (see Table 2-1), composite restorations have a greater change in dimensions with changes in oral temperatures than tooth structure. The more resin matrix, the higher is the linear coefficient of thermal expansion because the polymer has a higher value than the filler. As a result, microfilled composites have higher values for thermal expansion than microhybrid composites.
Water Sorption
Values of water sorption of microhybrid and microfilled composites are given in Table 4-2. The microfilled composites have a greater potential for being discolored by water-soluble stains. Water sorption is accompanied by swelling of the composite, but this has not been an effective way to counteract polymerization shrinkage. However, the effect of water sorption on degradation of properties of composites is irreversible.
Radiopacity
Most microhybrid composites are radiopaque. One microfilled composite (Heliomolar, Ivoclar Vivadent, Amherst, New York) contains ytterbium trifluoride, which makes it radiopaque. Most composites are radiopaque when compared with dentin but are radiolucent when compared with enamel. Argument exists whether radiopacity is an advantage in diagnosis; nevertheless, not all composites appear radiopaque on dental radiographs.
Compressive and Flexural Strengths
The compressive strength of microhybrid composites is higher than that of microfilled composites. Strength generally increases linearly with the volume fraction of filler. Because composite restorations most likely fail in tension or bending, their tensile and flexural strengths are of special interest (see Table 4-3).
Elastic Modulus
The elastic modulus, or stiffness, of the composites is dominated by the amount of filler and increases exponentially with the volume fraction of filler. The lower filler content of the microfilled composites results in elastic moduli of one fourth to one half of the more highly filled microhybrid composites. This stiffness is important in applications in which high biting forces are involved and wear resistance is essential. However, the rate of bond failure of Class V cervical restorations was higher for microhybrid composites when compared with microfilled composites; the lower modulus of the microfilled composites probably reduced the stress on the bond of the restoration to dentin. Values of elastic modulus in compression and bending are listed in Table 4-3.
Hardnes/>
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses