Biological and clinical rationale for root-canal treatment and management of its failure
Uncontrolled pulp disease (inflammation) spreads from the pulp chamber and main canals via the intercommunicating lateral canals and apical foramina to the periradicular tissues (Fig. 3.1); channels which normally serve to feed the pulp tissue with their neurovascular supply. While this apparently simple event of the inflammatory border passing the threshold of the tooth boundary may not seem that important, clinically, it is associated with a reduction in the success of root-canal treatment by 10–20%. It may well be that the critical boundary is that of the associated microbial front. Root-canal treatment is a therapeutic procedure used to prevent apical periodontitis when pulpal disease is considered too advanced to manage by vital pulp therapy but apical periodontitis is not yet established. Root-canal treatment is also a therapeutic procedure used to cure apical periodontitis when pulpal disease has advanced to its sequela, apical periodontitis.
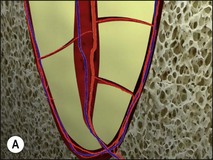
Fig. 3.1 (a,b) Spread of inflammation from the pulp chamber and main canals via the intercommunicating lateral canals and apical foramina to the periradicular tissues
The details of the treatment procedure are covered in Chapter 8, suffice it to say that it is a technically demanding protocol requiring a mastery and integration of tactile, spatial and cognitive senses. In a busy general practice, therefore, the performance of this feat holds sway over biological considerations in the dentist’s mind. The prevalence of apical periodontitis and the need for such treatment, worldwide, has underpinned the commercial development of instruments, materials and techniques to make root-canal treatment more efficient and putatively effective. However, the outcome data on periapical healing demonstrate that such technical and technological advances have not been matched by improvements in success (Fig. 3.2). As pointed out by others in the field of endodontology in the past, true advances cannot ignore biological considerations, either in the design of treatment strategy or its execution in practice.
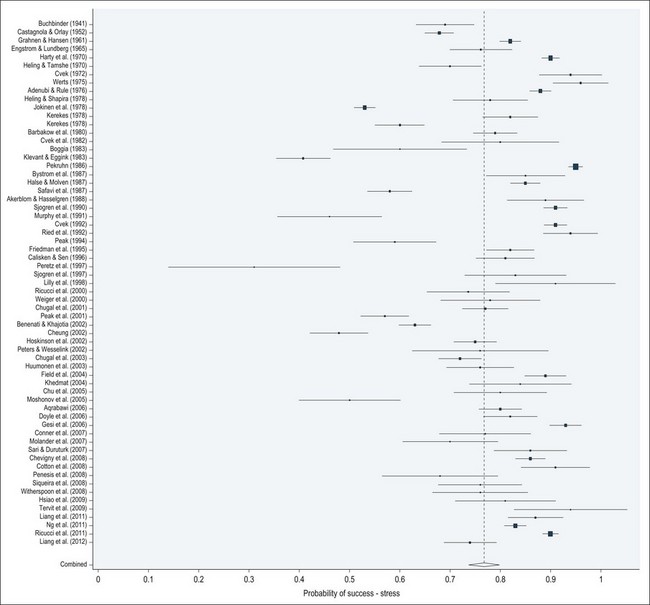
Fig. 3.2 Forest plot showing pooled and individual studies (1941–2012) probability of periapical healing following primary root canal treatment based on strict criteria
Aetiopathogenesis of periapical disease
Aetiological factors implicated
Initial uncertainty about the bacterial origin of periapical disease gave room for other theories to become established, including stagnant tissue fluid and necrotic pulp tissue. The former theory was conclusively disproved by Goldman and Pearson (1965). Histological studies have also shown growth of connective tissue and cementum-like hard tissue on root-canal walls after a 7-month exposure of apical tissues to necrotic pulp tissue.
Bacteria
The credit for demonstrating a definitive causal association between bacterial infection and periapical lesion development is extended to Kakehashi et al. (1965), who compared the pulpal and periapical reactions to experimental pulp exposure in germ-free and conventional rats. The teeth in the former case exhibited healing, while the latter showed pulp necrosis and periapical lesion development. The causal relationship between root-canal infection and periapical disease was further consolidated when Sundqvist (1976) recovered no cultivable bacteria from traumatized, intact teeth with an absence of periapical disease, while showing 18 out of 19 teeth associated with periapical disease to yield positive cultures.
Fungi
The presence of fungi in root canals has been reported in numerous studies (Table 3.1) and has been considered to be a potential cause of endodontic failure in root-filled teeth but definitive evidence is lacking. Various fungi (Candida, Aspergillus, Penicillium, Fusarium, Aureobasidium, Exophiala, Eurotium, Cladosporium) have been isolated from root canals.
Host factors implicated
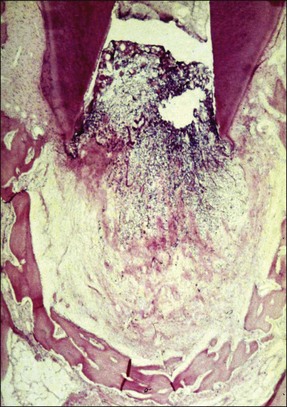
Periapical disease is the result of interaction between bacteria (or their products) and the host defences. Both the non-specific and specific branches of host defences are recruited to defend against the threat of invasion of the body by bacteria. The periapical lesion is, therefore, the retreat of the bone tissue away from the source of infection, creating space for the body’s defensive elements to migrate into the immediate vicinity of the infection to counter it (Fig. 3.3).
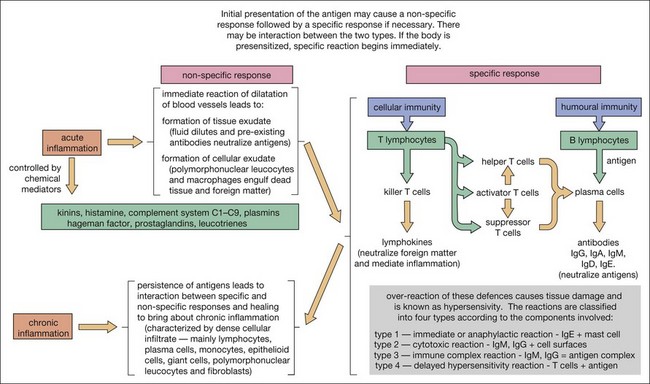
Knowledge of the composition of periapical lesions is based on histopathology of samples of long-standing lesions of unknown activity and often undefined clinical status. Nevertheless, the full range of immune mechanisms has been implicated in the development of the periapical lesion (Figs 3.4, 3.5).
The clinical course of the periapical lesion varies giving many presentations that have been classified into: acute apical periodontitis; chronic apical periodontitis; acute periapical abscess or suppuration (without sinus tract); chronic periapical abscess or suppuration (with sinus tract); and radicular cysts. The relative contribution of the normal inflammatory and immune responses varies in these clinical variants. The essential nature of the lesion (except in the incipient lesion) is chronic inflammatory (or granulomatous) tissue, meaning that the two principal elements, inflammation and attempts at healing are coexistent. Depending on the state of the lesion there may be a variable degree of tissue destruction, with the consequent concentration of polymorphonuclear leucocytes (PMNs) and macrophages in the vicinity of the apical foramina. The importance of the diagnostic category may lie in the role it may play in prognosticating on the outcome of treatment.
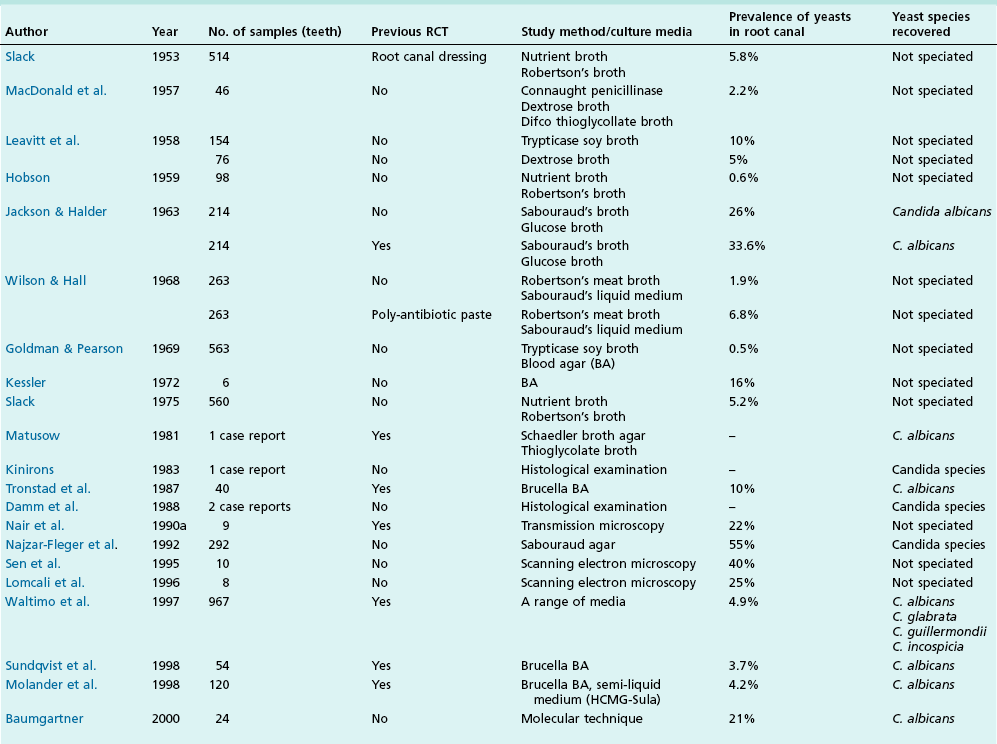
Chronic inflammation implies persistence of the irritant stimulus (bacteria and their products), in this case because of the protective sanctuary of the root-canal system, which the host defences can access only to a limited degree (Fig. 3.6). Therefore, in addition to the ubiquitous PMNs and macrophages, a small population of eosinophils and mast cells, various proportions of the immune (lymphocyte and plasma) cells are also evident. Epithelial cells may be present in variable proportions (up to 50%) as arcades of proliferating cells that may help to form a barrier across the apical foramina, as part of sinus tracts (Fig. 3.7a) or as the lining of developing cysts (Fig. 3.7b). Other tissue elements found in the periapical lesion include endothelial cells and fibroblasts, both being found where there are attempts at healing.
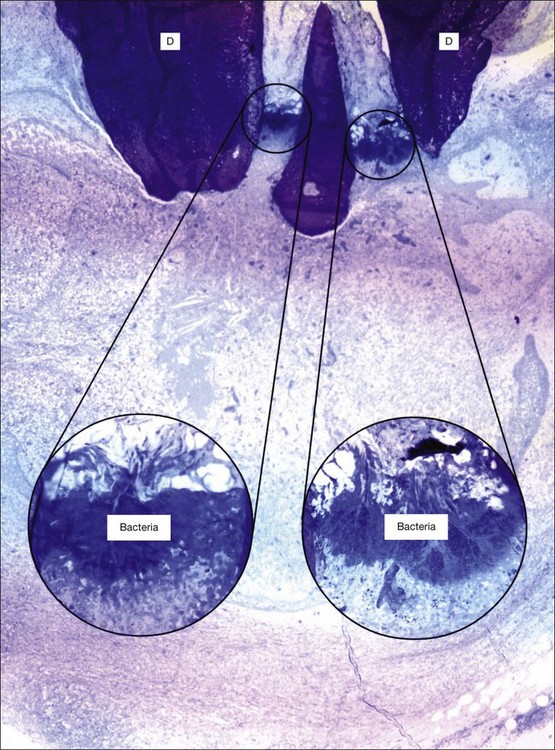
Fig. 3.6 Bacteria at the apical foramen of a tooth affected with apical periodontitis (D = dentine). The canal ramifications on the right and left, clogged with bacteria, are magnified in the circular insets. Note the strategic location of the bacterial clusters at the apical foramina. The bacterial mass appears to be held back by a distinct wall of neutrophilic granulocytes. Obviously, any surgical and/or microbial sampling procedures of the periapical tissue would contaminate the sample with the intraradicular flora: magnification: ×65, insets ×340 (courtesy of Dr PNR Nair)
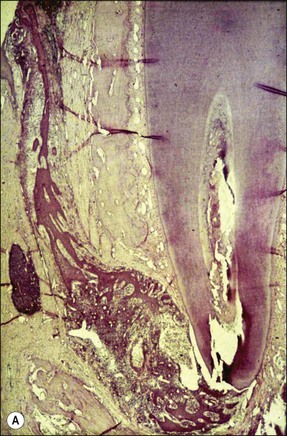
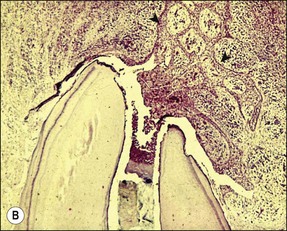
Fig. 3.7 (a) Epithelium-lined sinus tract (Valderhaug, 1974). (b) proliferation of epithelial tissue adjacent to the apical foramen
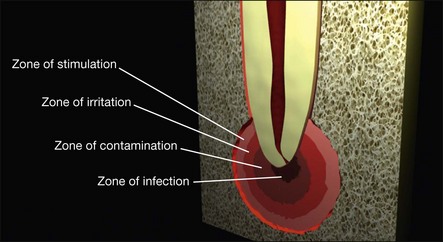
The three-dimensional distribution of these cellular elements is thought by some to fall into zones (Fig. 3.8), based on an animal model of bone infection, but others refute this concept, believing the cellular distribution to be too random for discrete zonal partition. Perhaps both views are correct, with zonal organization more evident in the pure chronic lesion and zonal disorganization brought about by repeated acute exacerbations.
Plasma cells have been demonstrated to be actively producing antibodies in peripical lesions. Members of the tumour necrosis factor family of receptors and ligands, receptor activator nuclear factor kappa B (NF-κB or RANK), ligand (RANKL) and osteoprotegerin (OPG), which are involved in bone metabolism, are found in increased levels when T and B lymphocytes and macrophages infiltrate chronic periapical lesions. The relative ratio of RANKL/OPG may be a key determinant of RANKL-mediated bone resorption (see Fig. 3.5).
The neural and immune systems also interact in controlling the non-specific and specific defensive responses and it is also possible that microorganisms participate in this regulatory function. The ultimate goal is to decipher the mechanisms involved so that a model can be constructed describing the entire sequence of interactions between the cellular (microbial and host) and neural components leading to the progressive development of the stable (in size) yet dynamic (in activity) periapical lesion. This requires synthesis of information from human and animal studies at both cellular and molecular levels. The mechanisms coordinating all these elements have not yet been fully elucidated, although it is known that some of them function as cascades.
The neuropeptides implicated in this process are calcitonin gene-related peptide (CGRP), substance P, vasoactive intestinal polypeptides, dopamine hydrolase, and neuropeptide Y. The most interesting among these is the possible role of CGRP, which has been implicated both in the response to pulp and periapical injury (Fig. 3.9).
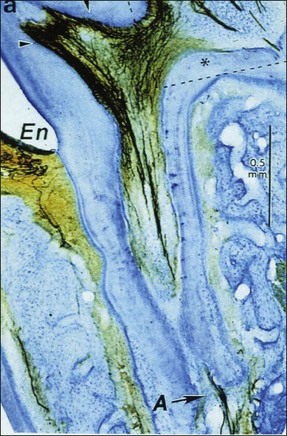
Fig. 3.9 Immunocytochemical section showing calcitonin gene-related peptide (CGRP) nerve fibres in pulp horns and also extending to the apical (A) part of the root canal: En = enamel (Byers et al., 1990)
A synthesized model of pathogenesis and natural history of periapical disease
The pathogenesis of periapical lesions has been studied in a variety of animal models by artificial induction. The models include rats, rabbits, dogs, ferrets, cats, and monkeys (Fig. 3.10a–c). The findings are interpreted with caution because the microbiota and host responses may be different from the human condition.
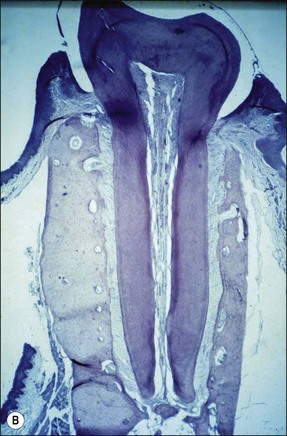
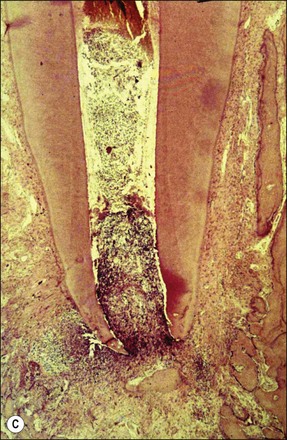
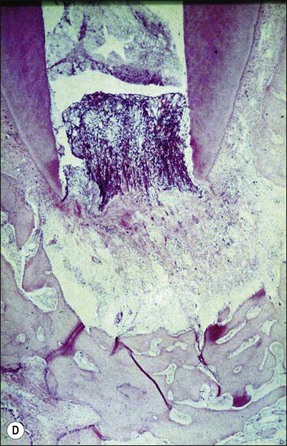
Fig. 3.10 (a) Monkey’s oral cavity used in experiments by Valderhaug (1974); (b) monkey model of apical periodontitis (Valderhaug, 1974); (c) apical progression of root canal infection and establishment of apical granuloma (Valderhaug, 1974); (d) localization of apical infection by a well-defined granuloma
As the root-canal infection develops and matures, it progresses apically in the root canal until certain, as yet undefined, elements, either bacterial products or bacteria themselves, are in a position to stimulate the periapical tissues via the apical foramina (Fig. 3.10c,d). In a host previously unexposed to the bacteria, the initial response is non-specific acute inflammation. This comprises first a fluid exudate induced by altered vascular permeability, which is mediated by several biochemical cascade systems consisting of preformed plasma proteins acting in parallel to initiate, propagate and control the inflammatory response (see Fig. 3.4). These include: (1) the complement system, which creates a cascade of chemical reactions that promotes opsonization, chemotaxis, and agglutination; it produces the membrane attack complex; (2) the kinin system, which generates proteins (e.g. bradyknin) capable of sustaining vasodilation and other physical inflammatory effects; (3) the coagulation system or clotting cascade, which forms a protective protein mesh over the sites of injury; and (4) the fibrinolysis system, which acts in opposition to the coagulation system, to counterbalance clotting and generate several other inflammatory mediators. Subproducts of these cascades may be responsible for further activation of the responses, enhancing the inflammatory process. In addition, products of metabolism such as arachidonic acid, also contribute to the production of proinflammatory mediators (leukotrienes and prostaglandins).
This is followed in rapid succession by a cellular exudate, consisting principally of PMNs, macrophages, dendritic cells and natural killer (NK) cells, which also release cell inflammatory mediators to augment the inflammatory process (see Fig. 3.5). The PMNs are attracted to the site by a number of bacteria-derived chemoattractants, such as the peptide F-Met-Leu-Phe that constitutes the amino terminus of many bacterial proteins, and other pathogen-associated molecular patterns (PAMPs) including components of lipopolysaccharides, mannose and teichoic acids. In a previously exposed host, these activate the innate immune response by interaction with different receptors known as pattern recognition receptors (PRR). The PRR, such as the family of Toll-like receptors (TLRs), are present mainly on macrophages, neutrophils, and dendritic cells (DCs). Other receptors present on phagocytes with an important role in the immune response are those for complement, cytokines, interleukins, and immunoglobulins.
The initial exudate will also bring with it any circulating specific antibodies that may be present. The PMN migration would also then be further stimulated by activation of complements C3b and C5a via antigen–antibody-complex formation. The overall effect of pre-existing immunity is better confinement of the bacteria to their source (apical foramina), requiring only a sparse cellular infiltrate to deal with them and a fibro-encapsulation of the rich granulation tissue (see Fig. 3.10d). In contrast, lack of pre-existing immunity results in poorer bacterial confinement as well as that of the cellular infiltrate, which is more diffuse and spread into the trabecular system. These histological pictures translate into radiographic views of well circumscribed, smaller, and better-demarcated periapical radiolucencies compared to more diffuse and extensive lesions, respectively. The acute response may be accompanied by the usual clinical signs of pain and tenderness on percussion of the tooth, which may feel elevated in the socket, but it may also be too transient and minimal to be noticed. So far, little bone resorption is likely to have taken place and little obvious radiographic change would be discernible (Fig. 3.11a).
Depending upon the nature of the stimulus and host susceptibility, a whole range of effects may be manifested, including Types 1–4 hypersensitivity reactions (see Fig. 3.4). The magnitude of the response varies from individual to individual, depending upon both the stimulus and the host characteristics. In some cases, the host response may be exaggerated causing greater damage than the bacterial assault. Other host-dependent factors, which are genetically determined, may also play a part in modulating the response but these are so far poorly studied for periapical disease.
The clinical progression of the lesion may take one of several paths, including: acute apical abscess formation, an intensely painful event until bone resorption relieves some of the tissue pressure; chronic suppuration with sinus tract formation; or conversion to a chronic, stable but dynamic state (see Fig. 3.11b).
Bacteria entering the periapical tissues would normally be removed rapidly by PMNs and macrophages; the latter release leukotrienes and prostaglandins. The former class of these molecules attracts more macrophages to the site and the latter contribute to bone resorption, creating space for invasion by more immune cells. The activated macrophages also continue to produce a variety of other mediators of inflammation, including IL-1, TNF-α, chemotactic factors such as IL-8, IL-10, IL-12, and interferon gamma (IFN-γ). These cytokines intensify the local vascular response, osteoclastic bone resorption, and can provoke a general alert by endocrine stimulation of fever and output of acute phase proteins and other serum factors. The IL-1 and TNF-α act in concert with IL-6 to upregulate the production of haemopoietic colony stimulating factors that are able to mobilize more PMNs and pro-macrophages from bone marrow. Death of bacteria, PMNs and macrophages in this encounter can result in suppuration, which may follow an acute or a chronic course (Fig. 3.12). In the latter case, cytokines and lipopolysaccharides from bacterial breakdown products may stimulate the epithelial cells in the rests of Malassez to proliferate and line a tissue path for pus to escape to a body surface (usually intraoral). Roughly half of all induced periapical lesions (in a monkey study) developed sinus tracts (see Fig. 3.7a). The relative prevalence of various clinical presentations in the unchecked disease process (natural history), that is the numbers that convert to acute or chronic abscesses (with or without sinus tracts) or become chronic asymptomatic granulomas, is unknown. It is likely that the majority follow an asymptomatic chronic progression.
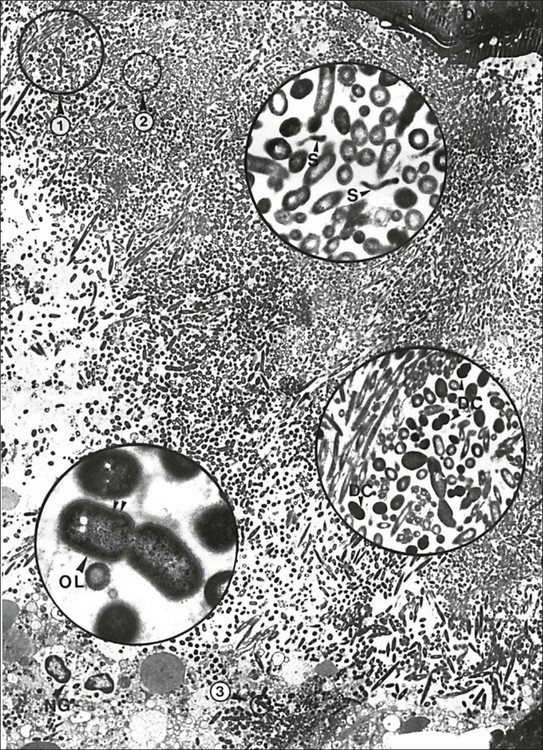
Fig. 3.12 A massive periapical plaque associated with an acute lesion. Note the mixed nature of the flora. Numerous dividing cocci (DC, middle inset), rods (lower inset), filamentous bacteria and spirochaetes (S, upper inset) can be seen. Rods often reveal a Gram-negative cell wall (double arrowhead), some of them showing a third outer layer (OL). The circular areas 1, 2 and 3 are magnified in the middle, upper and lower insets, respectively: D = dentine; C = cementum; NG = neutrophils. Original magnification ×2680; upper inset ×19 200; middle inset ×11 200; lower inset ×36 400 (from Nair, 1987)
An interesting phenomenon in chronic inflammatory periapical lesions is the observation that PMNs are chemoattracted to the apical foramina, where they congregate to form an almost continuous “barrier” to the egress of bacteria. In other cases, this barrier is formed by proliferation of epithelial cells by mechanisms already mentioned (Fig. 3.13).
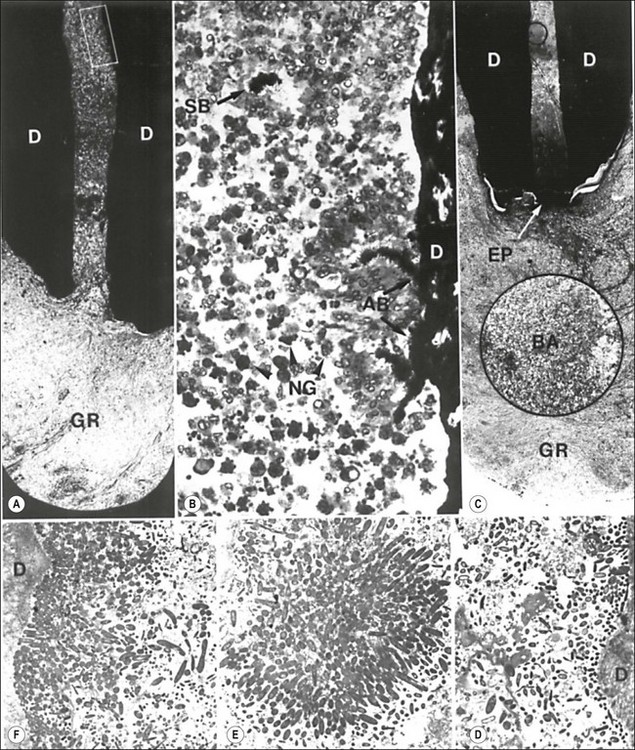
Fig. 3.13 The endodontic flora in the apical third of a periapically affected human root. The flora appears to be blocked by a wall of neutrophils (NG in b) or an epithelial plug (EP in c). Note the dense aggregates of bacteria sticking to the dentine wall (AB in b) and similar ones (SB in b) along with loose connections of bacteria (insert in c) remaining suspended in the root canal among neutrophils. A cluster of an apparently monobacterial colony is magnified in e. Electron micrographs show bacterial condensation on the surface of the dentine wall, forming thin (d) or thick (f) layered bacterial plaques. The rectangular demarcated portion in (a) and the circular one in (c) are magnified in (b) and the inset in (c), respectively: GR = granuloma, D = dentine. Original magnification: (a) ×50; (b) ×400; (c) ×40 (inset ×400); (d) ×2440; (e) ×3015; (f) ×3215 (from Nair 1987)
The chronic lesions may undergo acute exacerbations as a result of changes in the balance between the bacteria and host responses or due to the proliferation of specific bacteria. Such acute phases, accompanied by invasion of the periapical lesion by viable bacteria (many of which will die there) (see Fig. 3.12) may also result in increase in the size of the periapical lesion.
Acute periapical inflammation
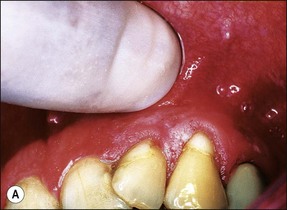
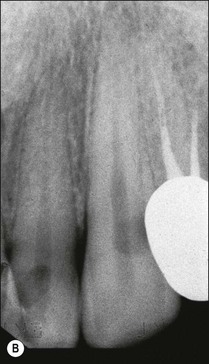
This is an uncommon presentation, but once clinically encountered is instantly recognizable and never forgotten. It arises when the transition from pulpal to periapical infection and inflammation occurs more rapidly than allowed by the process of periapical bone resorption. The result is an accumulation of PMNs and oedema due to the vascular response in the apical periodontal ligament, giving rise to severe pain. The tooth may feel raised in the socket, accompanied by acute tenderness to touch. At this early stage, there may not be any obvious periapical tenderness to palpation but it soon becomes apparent (Fig. 3.14a). There would be no demonstrable radiographic periapical change (Fig. 3.14b). Subsidence of the pain is accompanied by the appearance of a periapical area and the transition of the inflammation to a chronic state.
Chronic periapical inflammation
The histological picture of this entity is as described in detail above. It is clinically asymptomatic and presents as a radiographic periapical radiolucency (Fig. 3.15). The asymptomatic radiographic image masks a quiet dynamism and an array of histological variations in cell composition (described above) and epithelial proliferation with or without cystic change.
Epithelial proliferation and cysts
The epithelial rests of Malassez may be stimulated to proliferate by inflammatory mediators. The pattern of proliferation is variable, with the formation of strands, arcades or rings of epithelial clusters at the junction of the uninflamed connective tissue and granulation tissue (see Fig. 3.7b). Proliferation may also occur within the body of the granuloma, where it presumably helps to plug the apical foramina and limit egress of bacteria and their toxins (see Fig. 3.13). In some instances, these epithelial plugs bulge out into the periapical lesion, forming a sac connected to the root and continuous with the root canal, termed a “Bay or apical pocket cyst” (Fig. 3.16a,b). In these cases, microorganisms from the root canal have direct access to the “cyst” cavity and may invade it (Fig. 3.16c).
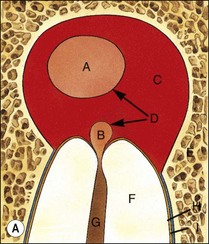
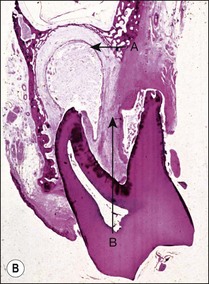
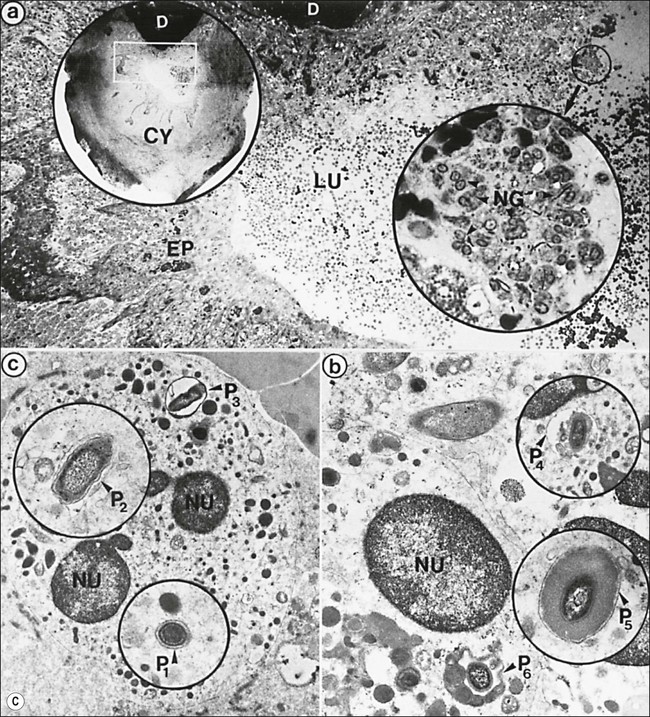
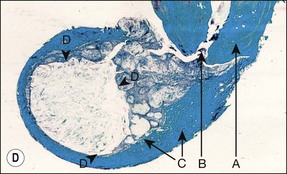
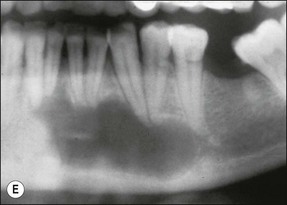
Fig. 3.16 (a) Bay and true cysts: A = true cyst; B = bay cyst; C = granuloma; D = epithelium; E = alveolar bone; F = dentine; G = root canal; H = cementum; I = periodontal ligament. (b) Histological section showing a bay cyst (A): B = granuloma;(c) Bacteria in a radicular cyst. Note the distinct epithelial lining (EP) of the cyst lumen (LU) and a cluster of neutrophils (NG) showing phagocytosed bacteria. The upper inset in (a) shows an overview of the well-encapsulated cyst (CY). The electron micrographs in (b) and (c) show the several types of membrane-delimited phagosomes (P1 to P6) containing bacteria. Note the close adherence of bacteria and the phagosome membrane in P1 and P2, although a clear space is visible between them in P3. An electron-dense coating of varying thickness may be distinguished on the bacterial surface in P4 and P5. Note the bacterium in P6 is devoid of such a coating but the phagosome contains several membrane-delimited granule-like structures: D = dentine; NU = nucleus. Original magnification (a) ×100: (left inset ×10, right inset ×850); (b) ×12 800 (upper inset ×8900, lower inset ×17 500); (c) (lower inset ×8900, upper inset ×17 500) (from Nair 1987). (d) Histological section of true cyst (D). A = root apex; B = root canal; C = granuloma. (e) Large periradicular cyst associated with mandibular incisors
A true cyst has been defined as a separate pathological, epithelium-lined cavity usually containing fluid or semi-solid material. It does not communicate with the root canal or any other opening (see Fig. 3.16a,d) and develops in periapical lesions. It was once believed that large circumscribed radiolucent lesions with a sclerotic border were likely to be cysts but such a correlation has not been proven. However, lesions of the size shown in Figure 3.16e are likely to show cystic change. The bay cyst should by inference heal by elimination of the bacterial contamination of the root canal. Successful treatment of the true cyst may require surgery if conventional root-canal treatment fails to resolve it. This supposition remains to be proven.
An alternative theory for genesis of cysts is that dividing epithelial cells grow until the central cells are starved of their nutrient source, causing their degeneration.
The method of cyst enlargement is also speculative. Theories involve selective absorption of fluids and an active biochemical interaction between the cyst wall and adjacent tissues. Whatever the final explanation, it is clear that cysts tend to grow and may be considered an independent pathological entity within another pathological entity, the granuloma (see Fig. 3.16d). The relative proportions of granulomatous and cyst tissue may vary. Although the reported prevalence of cysts among apical periodontitis lesions varies from 6% to 55%, meticulous serial sectioning and strict histopathological criteria show the actual prevalence of the cysts to be well below 20%. As such, it could be argued that while the granulomatous lesion may be treatable by removal of the aetiological agents from the canal, the cyst, having become independently established, may continue to flourish until specifically inhibited or disrupted.
Chronic suppurative periapical inflammation
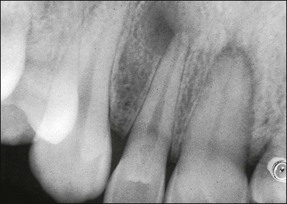
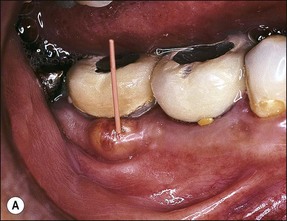
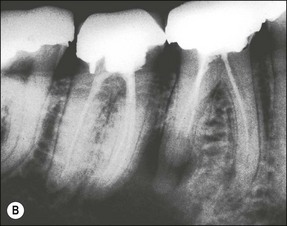
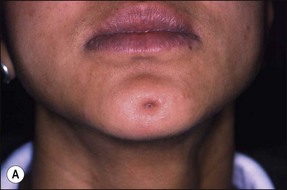
In some cases, the conflict between the bacteria and the defence cells, which are primarily the PMNs, results in the death of many bacteria and host cells without either side gaining the upper hand. The accumulation of dead cells and the consequent release of lysosomal enzymes results in the formation of pus. This is usually conveyed to the nearest body surface by the formation of a sinus tract, which over time, becomes lined by epithelium (see Fig. 3.7a). Clinically, there may be a draining sinus tract, which may sometimes be raised to form a “gumboil” (Fig. 3.17a) or rarely may drain extra-orally to the skin (Fig. 3.18). The patient may complain of bad taste in the mouth but rarely pain; there may sometimes be some mild discomfort especially on palpation adjacent to the involved tooth. The radiographic picture (Fig. 3.17b) would be similar to the previous category, however, the sinus tract presents the opportunity for placing a gutta-percha point in it, to trace its source if there is any doubt (Fig. 3.17a).
Acute periapical abscess/cellulitis
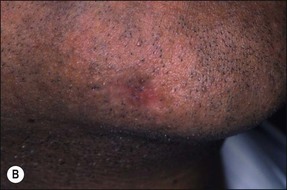
This entity may arise from any of the other categories described so far. When it arises directly from acute periapical inflammation without any other transition, it is an exquisitely painful condition. The pain usually subsides as soon as a periapical lesion forms and pressure is relieved. Opening access to such a tooth is difficult and is aided by applying pressure to the tooth with a finger while drilling, as vibration from the hand-piece causes the discomfort. The abscess is caused by an influx of large numbers of bacteria into the periapical area that overwhelm the defences (see Fig. 3.12). This causes a rapid influx of large numbers of PMNs. The rapid death of large numbers of cells and the release of lysosomal enzymes causes an accumulation of pus called an “abscess”. The highly acidic environment causes the further death of surrounding tissues and may lead to further exacerbation. Classically, it consists of a pathological cavity filled with pus and lined by a pyogenic membrane, which consists of granulation tissue. Clinically, there would be various degrees of swelling and pain (Fig. 3.19a,b) and the tooth would feel elevated in the socket. If severe, and accompanied by an allergic response, there is a large exudative component leading to a build-up of fluid pressure (Fig. 3.19c). The position of the swelling depends on the tissue planes (themselves determined by muscle and fascia attachments) through which pus spreads and accumulates (Fig. 3.19e–g). The lymphatic system is frequently involved in dental infections and gives an indication of the pattern of spread. Lymphadenitis, which comprises an enlargement and tenderness of the lymph nodes (Fig. 3.19d) may be present, as well as lymphangitis, characterized by an inflammation of the lymphatic vessels. The viable bacteria, cellulitis and pus may spread through the lymphatic system and along tissue planes formed by mucles and fascia, causing a spreading cellulitis with pyrexia, and the patient to feel unwell. The tissue space compartments where pus and exudate gather are described in detail in Chapter 10. Spreading cellulitis, typical of streptococcal microorganisms, manifests as a diffuse firm swelling and can lead to life-threatening conditions. If a maxillary tooth is involved, cavernous sinus thrombosis may develop where infection of the tissues of the face may spread intracranially via the interconnecting venous system, probably via the facial vein to the cavernous sinus. If a mandibular tooth is involved, Ludwig’s angina may be a risk. Prior to the antibiotic era, the mortality for Ludwig’s angina exceeded 50%, since the antibiotic era, and along with improved imaging and surgical techniques, mortality for the condition currently averages at 8%. Patients with Ludwig’s angina (Fig. 3.19h,i) become seriously ill, with marked pyrexia, and swallowing, speaking and breathing difficulties. If the glottis becomes involved the patient may die within 12–24 hours. The condition should be identified early and the patient referred urgently for medical attention (see details in Chapter 10).
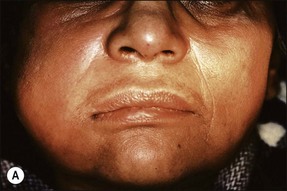
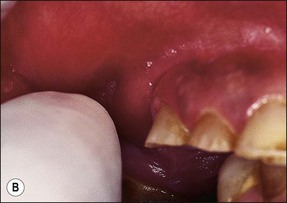
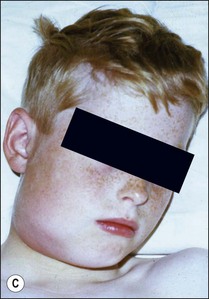
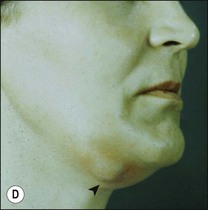
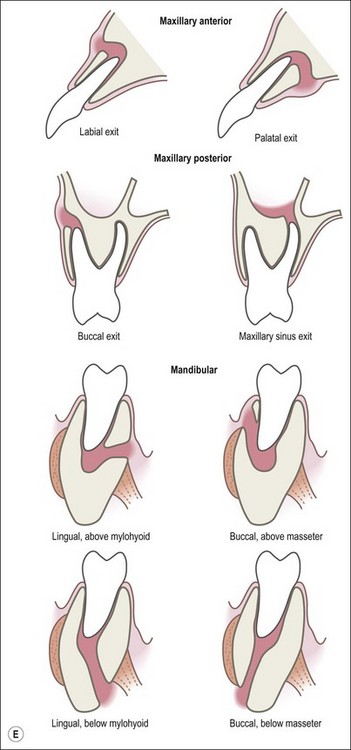
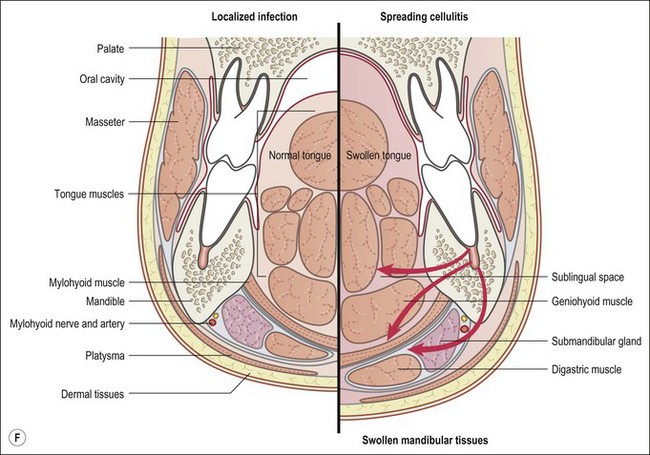
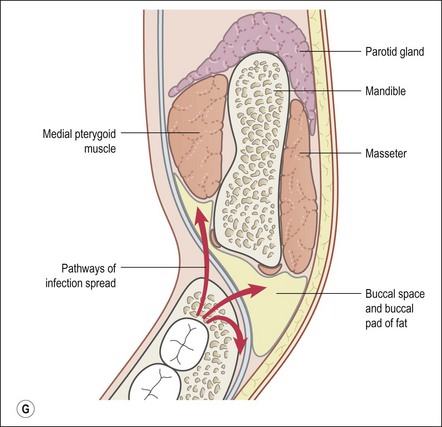
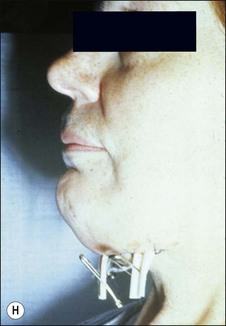
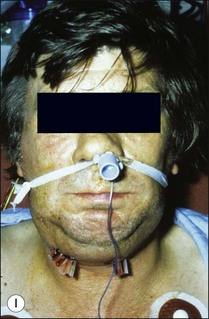
Fig. 3.19 (a) Right facial and infraorbital swelling associated with maxillary canine; (b) intraoral view of swelling in (a); (c) spreading submandibular swelling; (d) localized submandibular swelling (arrowed); (e–g) line diagrams showing the pathways of spread of infection; (h,i) treatment of Ludwig’s angina
Periapical osteosclerosis or condensing osteitis
Information is scarce on this relatively common presentation. It is thought to be a low-grade response of the body to mild irritation. A subdued response is caused by the buffering effect of intervening healthy tissue, for example, when the coronal part of a pulp is necrotic and infected while the apical portion is still vital. The intervening pulp tissue reduces the potency of the infection and its molecular products and, therefore, their direct impact on the periapical tissues. The effectively lowered dose stimulates bone deposition as opposed to resorption causing increased bone density with mild chronic inflammation in the marrow spaces. Clinically, the lesion is asymptomatic and presents as various grades of radiopacity surrounding a severely widened periodontal ligament space (Fig. 3.20). In rare instances, the inflammation is sufficient to cause apical root resorption at the same time (Fig. 3.21). The condition should not be confused with other radiopaque lesions, such as caused by benign tumours or dysplastic changes; 60% of radiopaque lesions are of periapical origin.
Nature of the periapical lesion associated with treated teeth
Much of the research on human periapical tissue has been conducted on undefined samples, that is, it is not known whether the sample was associated with treated or untreated teeth. Many investigators have assumed that the responses would be the same. Only a few studies have focused on specified tissue samples, either from treated teeth or untreated teeth. A quantitative comparison of lymphocytes and their subsets in periapical lesions harvested from treated or untreated teeth has shown differences in the inflammatory infiltrate and relative proportions of T, B and TH cells.
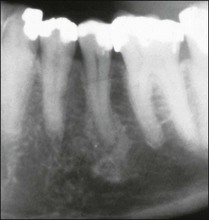
Periapical lesions persistent over a longer term may represent persistent chronic inflammation as a result of residual intraradicular infection (Fig. 3.22), established extraradicular infection (Figs 3.23, 3.24), a foreign body response (Fig. 3.25) or a radicular cyst (Fig. 3.26).
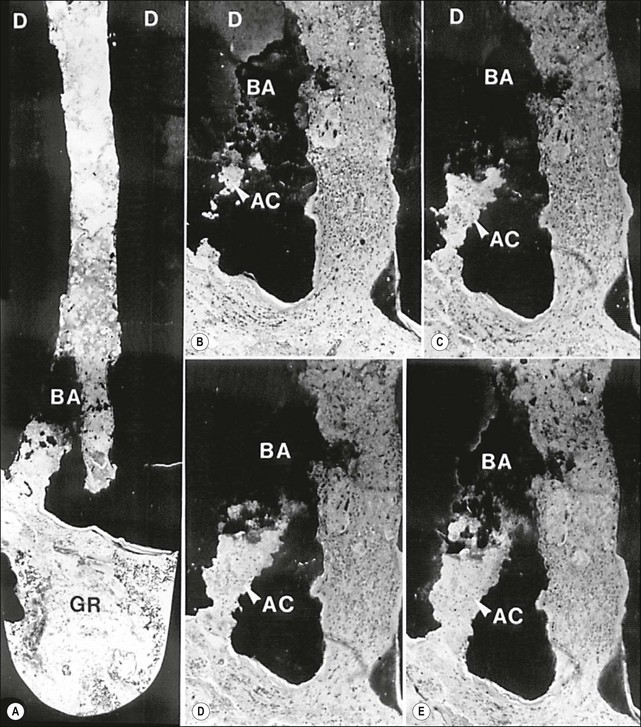
Fig. 3.22 Axial sections through the surgically removed apical portion of the root with a therapy-resistant periapical lesion (GR). Note the cluster of bacteria visible in the root canal (BA). Parts (b–e) show serial semi-thin sections taken at varying distances from the section plane of (a) to reveal the emerging (b) and gradually widening (c–e) profiles of an accessory root canal (AC). Note that the accessory canal is clogged with bacteria (BA). Original magnification: (a) ×52; (b–e) ×62 (from Nair et al., 1990)
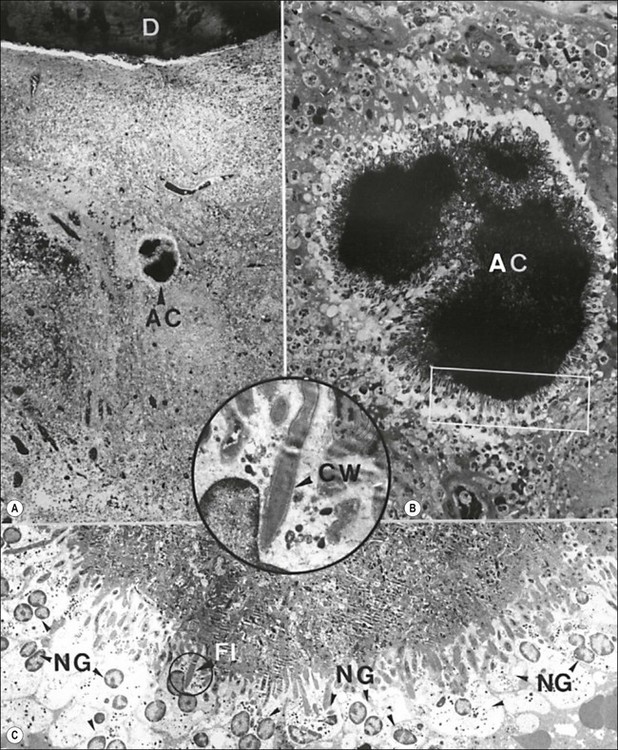
Fig. 3.23 Actinomyces in the body of a human periapical granuloma. The colony (AC in a) is magnified in (b). The rectangular area demarcated in (b) is magnified in (c). Note the starburst appearance of the colony with needle-like peripheral filaments surrounded by few layers of neutrophilic granulocytes (NG), some of which contain phagocytosed bacteria. A dividing peripheral filament (FI) is magnified in the inset. Note the typical Gram-positive wall (CW): D = dentine. Original magnification: (a) ×60; (b) ×430; (c) ×1680; inset ×6700 (from Nair & Schroeder 1984)
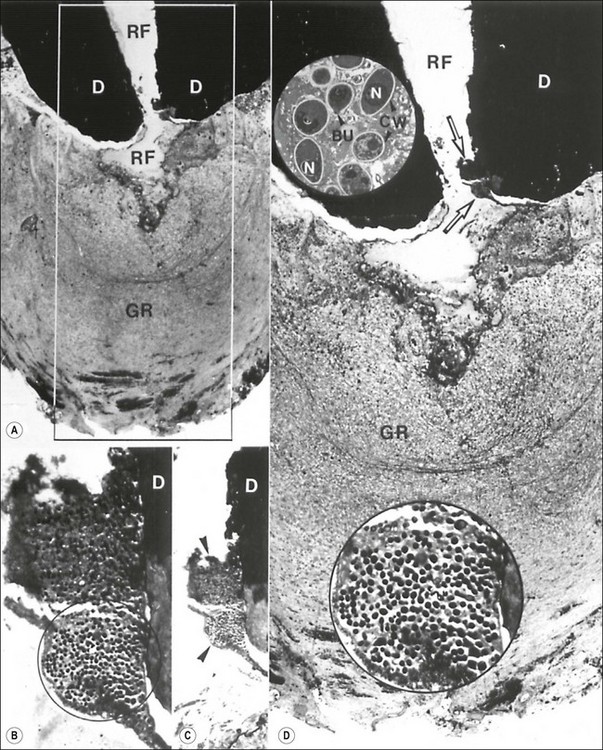
Fig. 3.24 Fungus in the root canal and apical foramen of a root-filled (RF in a and d) tooth with a therapy-resistant periapical lesion (GR in a and d). The rectangular demarcated area in (a) is magnified in (d). Note the two clusters of microorganisms located between the dentinal wall (D) and the root filling (arrows in d). Those microbial clusters are stepwise magnified in (c) and (d). The circular demarcated area in (b) is further magnified in the lower inset in (d). The upper inset is an electron microscopic view of the orgnisms. They are about 3–4 µm in diameter and reveal distinct cell wall (CW), nuclei (N) and budding forms (BU). Original magnifications: (a) ×33; (b) ×330; (c) ×132; (d) ×59; lower inset × 530; upper inset × 3400 (from Nair et al., 1990a)
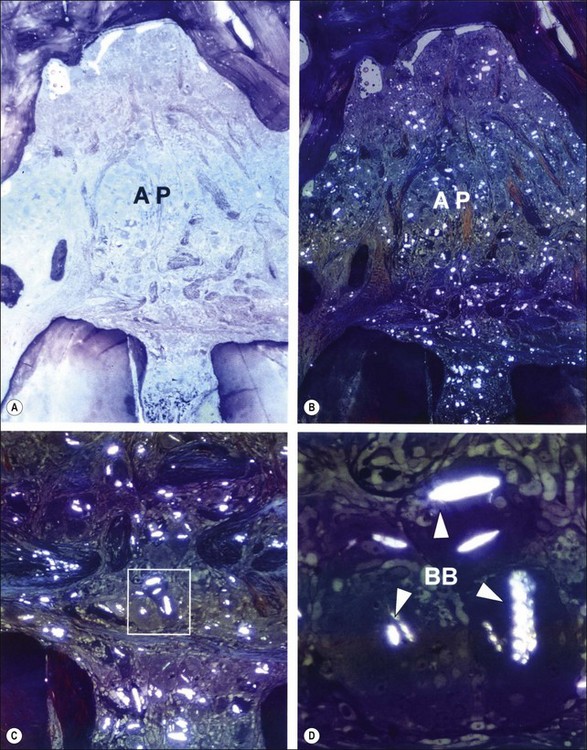
Fig. 3.25 Apical periodontitis (AP) characterized by foreign body giant cell reaction to gutta-percha cones contaminated with talc (a). The same field when viewed in polarized lights (b). Note the birefringent bodies distributed throughout the lesion (b). The apical foramen is magnified in (c) and the rectangular demarcated area in (c) is further enlarged in (d). Note the birefringence (BB) emerging from slit-like inclusion bodies in multinucleated giant cells. Magnifications: (a, b) ×25; (c) ×66; (d) ×300 (from Nair, 1998)
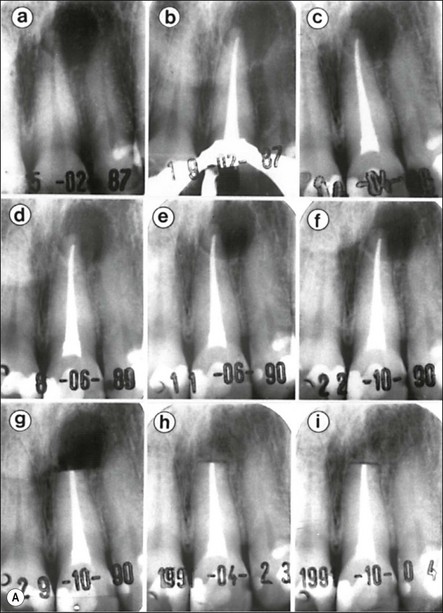
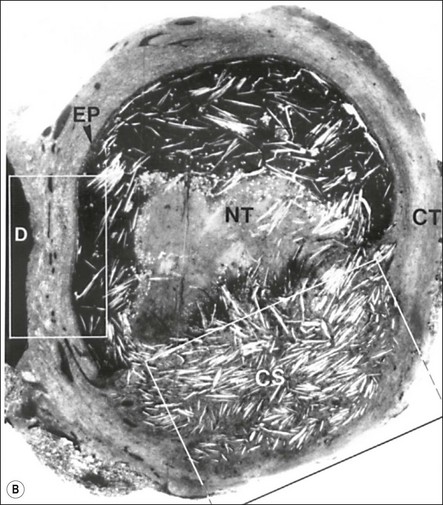
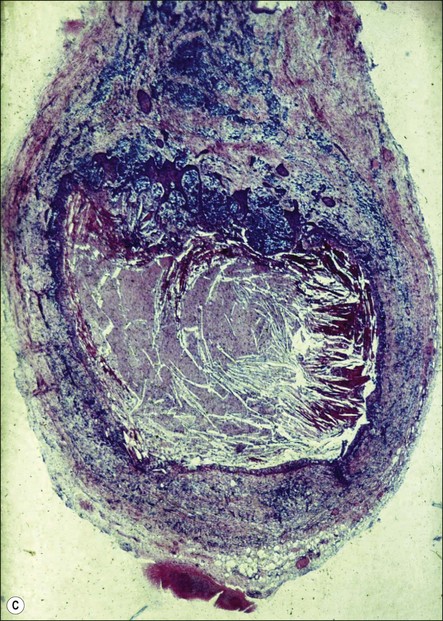
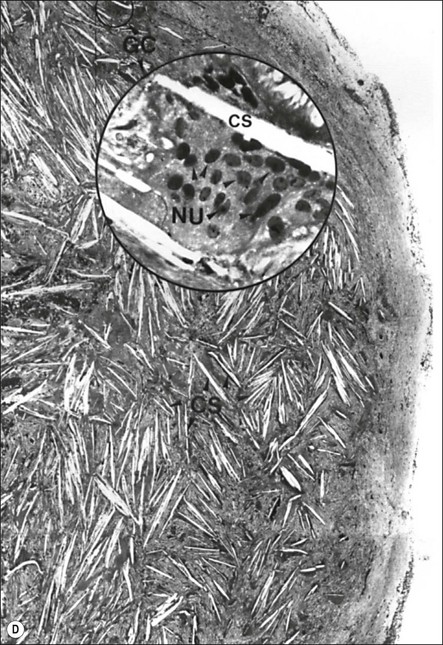
Fig. 3.26 (a) Longitudinal radiographs of a periapically affected left central incisor of a 37-year-old woman over a period of 4 years and 9 months of clinical management. Note the large eccentrically located apical radiolucency observed before (a) and immediately after (b) root filling. The lesion did not show any reduction in size in control radiographs taken 14, 28, 40 and 44 months (c–f) after endodontics. Apical surgery was performed (g) and the periapical area shows distinct bone healing (h,i) within 1 year of surgery (from Nair et al., 1993). (b) Axial section through the apical biopsy removed from the radiolucent area visible in (a) & (g). The large lesion is encapsulated with a narrow rim of dense capsular connective tissue (CT) and contains a distinct lumen lined with stratified squamous epithelium (EP). Note the vast number of cholesterol clefts (CS) concentrated in the connective tissue at the distocervical aspect of the lesion. The luminal centre contains pale staining necrotic tissue (NT) and the rest of the lumen is filled with dark staining erythrocytes, among which cholesterol spaces can be seen. The large rectangular demarcated area is further magnified in (d) (from Nair et al., 1993). (c) Section of a true apical radicular cyst. (d) Presence of vast numbers of cholesterol clefts (CS) in the lesion. The cholesterol spaces are surrounded by multinucleated giant cells (GC), of which a selected one is magnified in the inset. Note the large number of nuclei (NU). Original magnification ×98 (inset ×322) (from Nair et al., 1993)
Association between root-canal microbiota and periapical lesion development
Animal investigations found changes in the root-canal microbiota to be associated with development of the periapical lesion. During the critical period of periapical lesion expansion (between days 7 and 15), the bacterial load did not increase but the proportion of strict anaerobes and the proportion of Gram-negative bacteria doubled. The mean number of cultivable species (≈3.5) per tooth remained the same during lesion development but the overall diversity increased on day 15. Therefore, the critical period of lesion expansion correlated with the root-canal microbiota becoming more anaerobic and Gram-negative without increase in total cell numbers.
To account for the possible contribution of unsampled or uncultivated bacteria in the pathogenesis of lesions, eleven isolated strains (including eight strains from one tooth, representing its total cultivable infection) were inoculated in freshly necrotized monkey teeth, in various combinations, but always in equal proportions. After 6 months, the “eight-strain collection” (Prevotella oralis [formerly Bacteroides oralis], Fusobacterium necrophorum, Fusobacterium nucleatum, Streptococcus milleri, Enterococcus faecalis [formerly Streptococcus faecalis], Peptostreptococcus anaerobius, Actinomyces bovis and Propionibacterium acnes) was recovered from all teeth but in the same proportions in which it had been recovered from the original tooth. This strongly suggested that selective pressures were at play in the root-canal system to reproduce the “same infection”. Other combinations did not survive as effectively, while some species were not recovered at all, suggesting that the cultured isolates were the main core of the functional microbiome.
Despite several species being implicated by association with symptomatic apical periodontitis (Table 3.2), a cause–effect relationship remains elusive. Black-pigmented Gram-negative anaerobic rods (Black-pigmented Bacteria; BPB), especially Prevotella melaninogenica (formerly Bacteroides melaninogenicus), as well as Parvimonas micra (formerly Peptostreptococcus micro) were originally implicated in purulent infections. The presence of black-pigmented Gram-negative anaerobic rods does not, however, consistently result in acute abscesses. Other species have also become associated with the presence of symptoms, including Actinomyces species, Finegoldia magna (formerly Peptostreptococcus magnus), non-pigmented Prevotella and Porphyromonas species, Peptococcus species, Eubacterium species, Propionibacterium species, spirochaetes; and Fusobacterium species (Table 3.2). Given the ecological basis for infections, it is likely that the cause of symptomatic exacerbation is complex and related to the number of bacterial cells, strain variants, nature of interaction between species/strains, and other as yet unknown microbial factors. Since the outcome is also dependent upon interaction between the microbiota and the host, compromising factors such as viruses could conceivably play a part, as may other immune burdens. Although the true basis for acute exacerbation of periapical lesions is still unclear, molecular studies comparing acute and chronic infections have suggested that some strains are associated with acute cases more than chronic cases. More recently, pyrosequencing, which allows massive parallel sequencing, has suggested the existence of specific consortia related to location in root canals, disease severity and clinical presentation. The most abundant phyla in acute infections were Fusobacteria with predominance of the genera Fusobacterium and Parvimonas.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses