Principles of Pharmacology
Learning Outcomes
On completion of this chapter, the student will be able to achieve the following objectives:
• Pronounce, define, and spell the Key Terms.
• Differentiate between a drug’s chemical, generic, and brand or trade names.
• List each part of a prescription.
• Describe the use of drug reference materials.
• Describe the stages a drug goes through in the body.
• Describe how medications are administered.
• List the commonly prescribed drugs in dentistry.
• List the commonly prescribed drugs in medicine.
• Describe the negative effects of drug use.
• Cite relevant factors in determining the dosage of a drug.
Electronic Resources
![]() Additional information related to content in Chapter 30 can be found on the companion Evolve Web site.
Additional information related to content in Chapter 30 can be found on the companion Evolve Web site.
Key Terms
Absorption Process by which the body takes in or receives (absorbs) a drug.
Distribution Action by which a drug is released throughout the body.
Dosage Amount of drug to be administered in a specific time, often according to body weight.
Dose A specified quantity of a drug or medicine.
Drug A substance used in the diagnosis, treatment, or prevention of a disease.
Ethical drug A drug that requires a prescription.
Excretion (ek-SKREE-shun) Action by which a drug leaves the body.
Generic (jeh-NAIR-ik) Drug sold without a brand name or trademark.
Inscription The name and quantity of a drug listed on the prescription.
Metabolism (me-TAB-eh-liz-um) Physical and chemical processes that occur within a living cell or organism and are necessary for the maintenance of life.
Patent medicine Drug that can be obtained without a prescription; also called over-the-counter drug.
Pharmacology A branch of medicine concerned with the uses, effects, and actions of drugs.
Prescription A written order for a specific drug.
Prophylaxis (pro-fah-LAK-sus) Administration of drugs to prevent disease or protect a patient.
Signature Instructions on a prescription explaining how to take a specific medicine.
Subscription Directions to the pharmacist for mixing the medication; this is seldom done by the pharmacist anymore.
Superscription The patient’s name, address, date, and Rx symbol on a prescription.
Systemic (sis-TE-mik) Referring to a drug that affects a specific system (or multiple systems) of the body.
Pharmacology is the science or branch of medicine that includes the research, development, and manufacture of drugs. A drug is a substance that can be taken for the prevention, diagnosis, and treatment of a disease. All drugs must be recognized and defined by the U.S. Food, Drug, and Cosmetic Act before they can be marketed for public use in the United States.
A vast variety of drugs are available on the market, and each drug produces a different effect. The most probable situations where the dental assistant will be required to identify drug types include (1) when reviewing a patient’s medication history; (2) when assisting in dental procedures that require premedication; (3) when assisting in a specific dental procedure for which pain control is required; and (4) when assisting in a medical emergency.
Your role in understanding pharmacology is to become familiar with the drugs used in dentistry, the drugs your patients are taking for a specific medical condition, the terminology and use of prescriptions, and the drug reference materials that are available.
Overview of Drugs
Drugs are derived from many sources. Organic drugs are derived from living organisms such as plants or animals, and inorganic drugs are synthesized in the laboratory or extracted from inorganic compounds. Most drugs today are derived from a chemical source, which makes them more pure in form than those derived from an original natural source that may be contaminated or polluted. The manufacturing of drugs takes place in a pharmaceutical laboratory.
A drug can be identified by three names:
Dispensing of Drugs
Drugs are classified into two categories according to the way an individual can purchase them: patent medicines and prescription drugs. Patent medicines are drugs that can be obtained without a prescription; these are also referred to as over-the-counter drugs (OTC). The U.S. Food and Drug Administration (FDA) regulates the sale of patent medicines and evaluates their safety and effectiveness for daily use. Prescription drugs, also termed ethical drugs, are licensed medicines that are regulated by legislation; a prescription is required before they can be obtained from a pharmacist. An understanding of the term ethical is important in that these types of drugs can be harmful to the patient if they are not used correctly (i.e., it would not be ethical to prescribe or supply these drugs improperly). A patient who is taking a prescribed drug must be under the guidance of a physician or dentist.
Controlled Substance Act
The drugs and drug products covered under the Federal Comprehensive Drug Abuse Prevention and Control Act are divided into five schedules. The drugs included in this Act, also referred to as schedule drugs, are classified on the basis of their potential for abuse, their medical usefulness, and the extent to which they may lead to physical and psychological dependence.
Many individual states have their own controlled substances acts, which are patterned after the federal law. Some state laws are more restrictive, but none may be less restrictive than the federal law. The dentist must comply with the provisions of the federal law and those of the state in which he or she practices (always following the most restrictive guidelines when the laws differ). Under these laws, any professional who is authorized to prescribe these medications is issued a Federal Drug Enforcement Agency (DEA) identification number, which is to be printed on the dentist’s prescription pad.
Schedule I Drugs
Schedule I drugs have no current accepted medical usefulness and have a high potential for abuse. Normally, Schedule I drugs cannot be prescribed. This group includes certain derivatives (such as heroin), hallucinogenic substances (such as LSD and marijuana), depressants, and stimulants.
Schedule II Drugs
Schedule II drugs, although they have a high potential for abuse, have accepted medical usefulness. Prescriptions for these drugs are given in writing and cannot be renewed. Schedule II drugs include opium and opium derivatives, cocaine, morphine, ritalin, adderall, oxycodone, methadone, and barbiturates.
Schedule III Drugs
Schedule III drugs have less abuse potential than the drugs in Schedules I and II and also have accepted medical usefulness. Prescriptions for Schedule III drugs may be renewed. Schedule III drugs include some stimulants and depressants; the combination of Tylenol with codeine is an example of a common prescription included in this schedule. Examples of other Schedule III drugs are steroids, PCP, and THC.
Schedule IV Drugs
Schedule IV drugs have low abuse potential and have accepted medical usefulness. Prescriptions for Schedule IV drugs may be renewed. A patient may have up to five refills of these drugs in a 6-month period. Examples of Schedule IV drugs include chlordiazepoxide (Librium), diazepam (Valium), and propoxyphene (Darvon).
Schedule V Drugs
Schedule V drugs have the lowest abuse potential and have accepted medical usefulness. Some states do require that these drugs be dispensed only by prescription. Under federal law, however, they are available only under controlled circumstances. Examples of Schedule V drugs include cough medicines with codeine. With the misuse and illicit distribution of methamphetamine (pseudoephedrine), many states have passed a law requiring a prescription, or requiring customers to show a photo ID and sign a log book, before pharmacies can dispense any cold remedy containing pseudoephedrine.
Prescriptions
A prescription is a written order provided by a physician or dentist for the preparation and administration of a medicine. Only a professional who is legally authorized to prescribe medications may write a prescription. A professional who is authorized to prescribe medications is issued a DEA identification number.
Under no circumstances can any member of the dental team prescribe medications, except the dentist. The dental assistant may dispense medicine according to explicit instructions and under direct supervision of the dentist.
Prescription Terminology
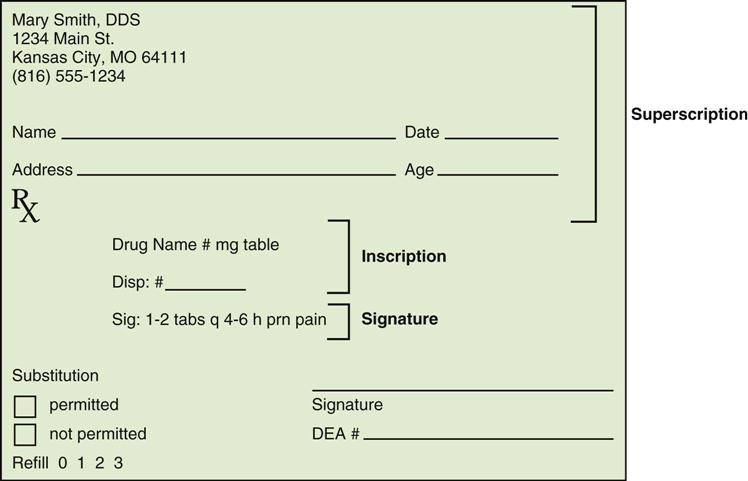
Prescriptions are handwritten on preprinted prescription forms that are assembled into pads. Prescription pads should be kept in a locked drawer and should never be visible or available to use as notepaper. Individual state laws regulate the format and information to be included on a prescription. In general, however, all prescriptions include the following four parts with their commonly used abbreviations (Fig. 30-1 and Table 30-1):
• Superscription: patient name and address, the date, and the symbol Rx (Latin for “recipe”)
• Inscription: name and quantity of the drug
• Subscription: directions for mixing the medication (this is now completed by the pharmacist)
TABLE 30-1
Common Prescription Abbreviations
| Abbreviation | Meaning |
| a.a. | of each |
| a.c. | before meals |
| a.m. | Morning |
| b.i.d. | twice a day |
| disp. | Dispense |
| H | Hour |
| h.s. | at bedtime |
| NPO | nothing by mouth |
| p.c. | after meals |
| prn | As needed |
| q. | Every |
| q.d. |
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses