3
The Blood Leukocytes
In this chapter we are going to be dealing with white blood cells (leukocytes) and their role within the tissue as part of the inflammatory response. At long last you are going to inspect the troops! We will meet the “Special Forces” and find out about their individual training, development, and domains of expertise. Hang on to your helmets . . . there are those who consider this the really bloody part of the defense system.
The individual leukocytes
The battle plan (code name “exudation of neutrophils”)
In this section we decided to bring a lot of material covered so far into an overall view or “battle plan.” We ve described the transport and delivery system and exudation, so now we will look at how neutrophils leave the bloodstream and enter the tissues. We ve discussed some of the mediators and their roles in the setup, so we will look at the actual attack by the neutrophils (phagocytosis). We will also review the potential outcomes of battle and how the local terrain can be changed and altered by the forces brought to bear, and sometimes, how the local population is hurt by the warring factions (tissue necrosis). Each of the following events will be covered:
- Margination (coming into contact with the vessel wall)
- Adhesion (sticking to the endothelial surface)
- Emigration (leaving the bloodstream)
- Chemotaxis (moving toward the target)
- Opsonization (recognizing and attaching to the target)
- Phagocytosis (engulfing of the target)
- Killing (zapping the target)
- Digestion of the victim (hey, we warned you these guys are tough!)
Why is it that neutrophils accumulate at a site of injury? Where do the marching orders come from? The following sequence seems to explain the phenomenon:
1. Margination
As stasis of the blood within the postcapillary venules occurs, masses of red blood cells clump together and occupy a central position within the axial stream. This is termed “rouleaux” formation. Simultaneously, the blood leukocytes move to the periphery of the venule. This is referred to as leukocyte margination. Subsequently, the neutrophil adheres to the endothelial surface (Fig 3-1). Corticosteroids and alcohol reduce adherence.
2. Adhesion
What causes neutrophils to adhere to the endothelial surface? This involves interplay between specific adhesion molecules present on the surfaces of neutrophils and endothelial cells (Fig 3-2). Three such proteins on the surface of endothelial cells are endothelial leukocyte adhesion molecule-1 (ELAM-1), intracellular adhesion molecule-1 (ICAM-1), and GMP-140. There are other adhesion molecules present on neutrophil surfaces (eg, LFA-1, MO-1, and p-150). Certain inflammatory mediators such as IL-1, tumor necrosis factoralpha (TNF-α), chemotactic factors, and histamine stimulate the activation of these adhesion molecules.
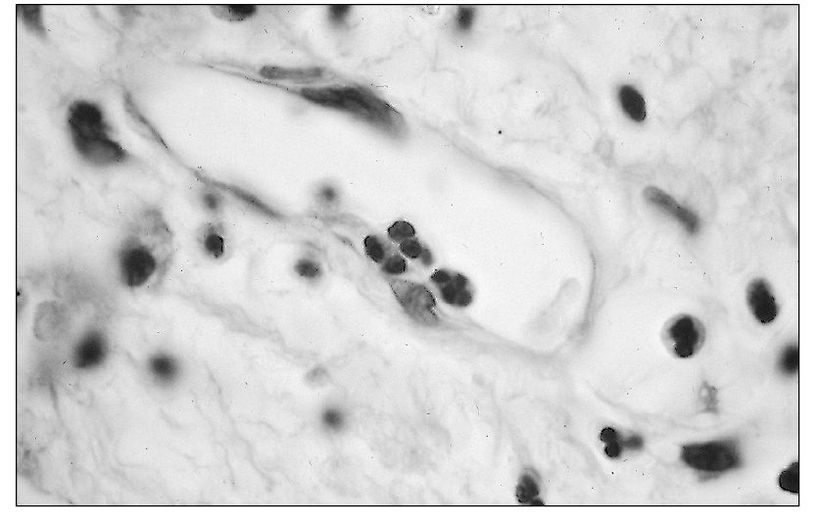
Fig 3-1 Adhesion of a neutrophil to the endothelial lining of a small venule.
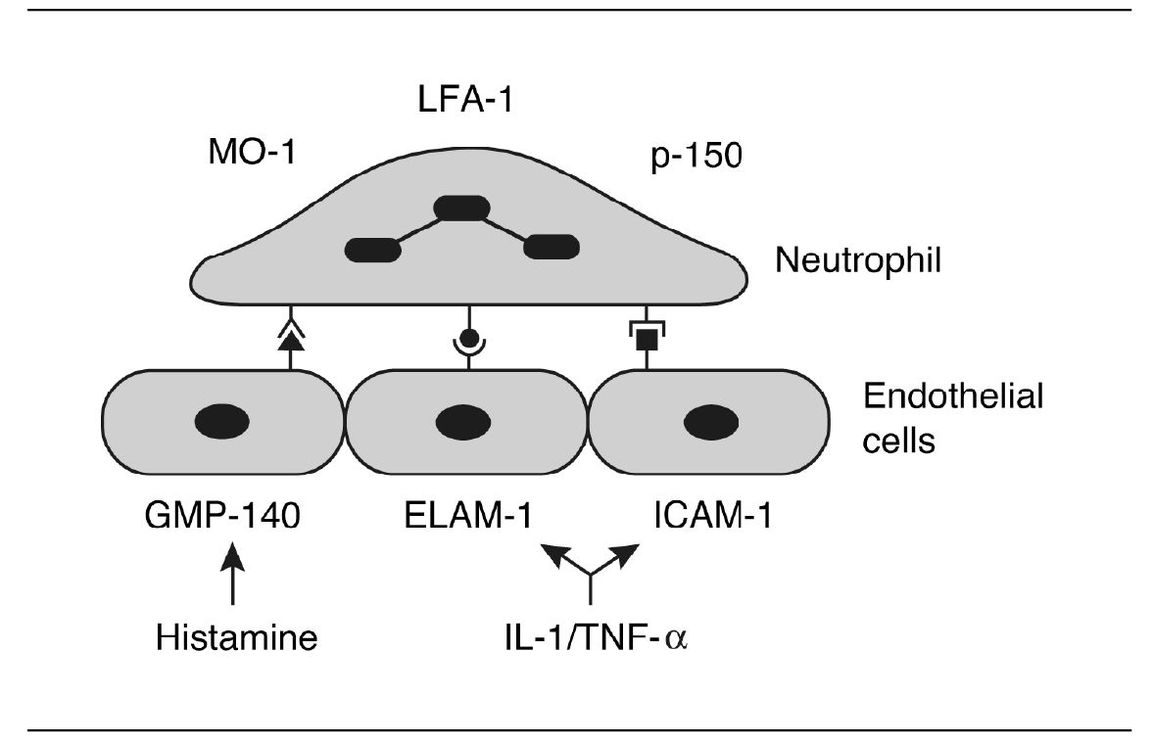
Fig 3-2 Binding of leukocyte to receptors expressed by endothelial cell.
Some of the chemotactic factors to be discussed later are also involved in adhesion (eg, C5a, leukotriene B4).
3. Emigration
Following adherence, neutrophils emigrate out of the venule. The cell forces its way out by thrusting a pseudopod through the intercellular junction between endothelial cells and then squeezing through by ameboid movement (Fig 3-3). Pseudo-podia form at the advancing edge of the neutrophil. Actin filaments in the microtubules and microfilaments are responsible for movement.
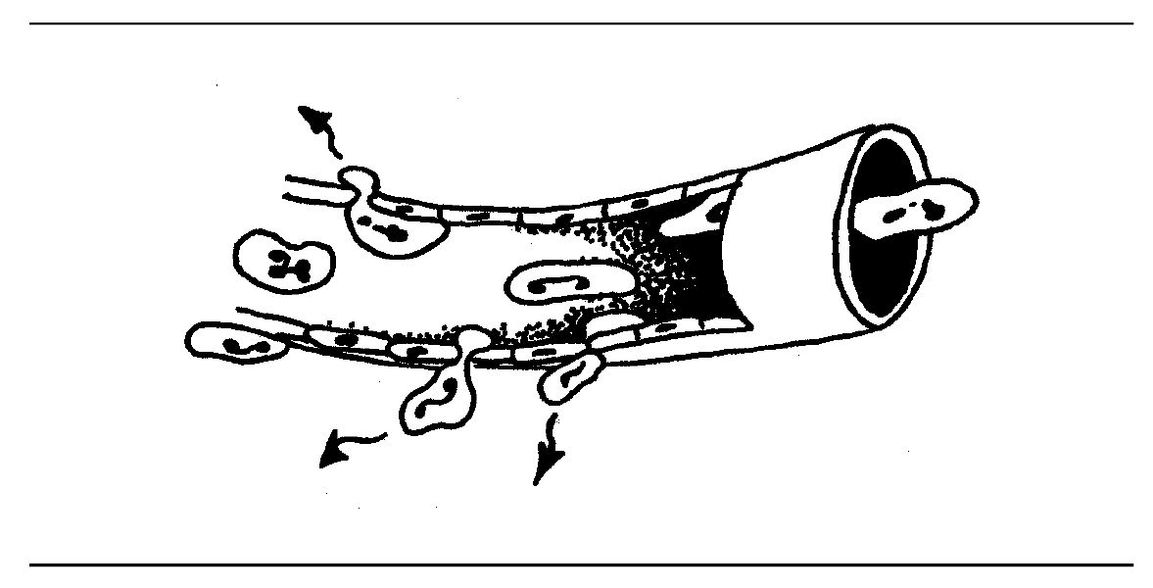
Fig 3-3 Leukocyte emigration.
Emigration of neutrophils occurs in the venules rather than arterioles and capillaries. There is a relationship between venular permeability and emigration in that some permeability mediators promote emigration, and some leukocyte products directly or indirectly enhance venular permeability.
4. Chemotaxis
Emigration and subsequent migration of neutrophils through the tissue are due to chemotactic factors (Fig 3-4). The general characteristics of these factors are:
- They stimulate the direction of migration.
- They are able to combine with receptor sites on leukocyte cell membranes.
- They are water soluble and therefore diffusible.
- They are usually small proteins (peptides).
- They may be of endogenous or exogenous origin.
When we last saw our friends C3a, C4a, and C5a they were floating along in space. They were busily causing histamine release. Well, C5a can do another amazing thing, it can cause white blood cells to move toward a site of injury.
This is called chemotaxis. Chemotaxis enables neutrophils to locate and destroy their prey. In this way, invading microorganisms can be phagocytosed and killed.
What other substances can activate chemotaxis? We tend to classify chemotactic factors as being either complement-dependent or complement-independent. Complement-depen-dent factors activate complement, and this results in the formation of fragments C5a and C567.
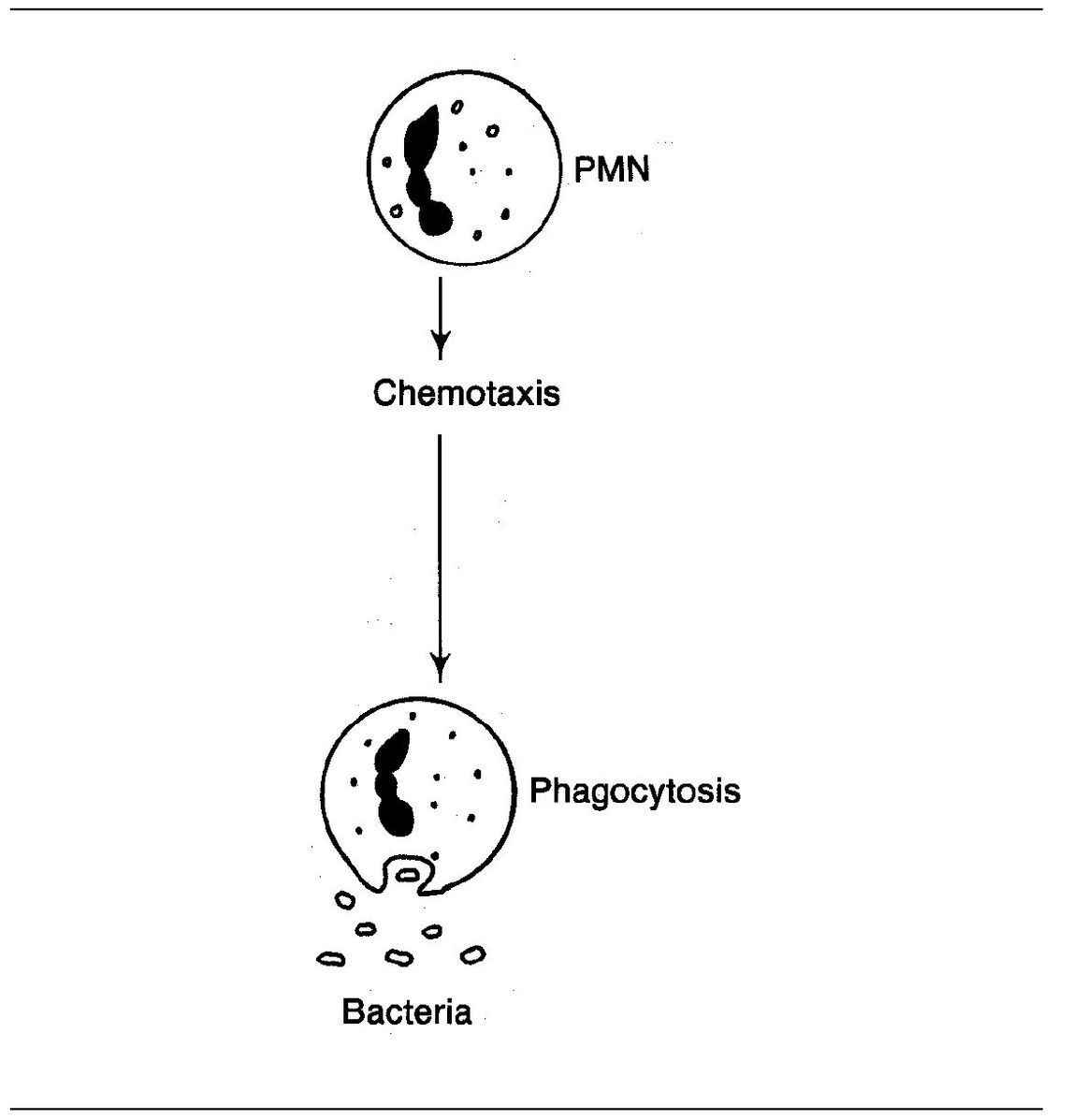
Fig 3-4 Chemotactic action.
Complement-independent factors affect cells directly without activating the complement system. For example, LTB4 is an important chemotactic factor for neutrophils. Many of these factors are small soluble proteins (low-molecular weight N-formylated peptides) that are products of bacterial metabolism. Other bacterial products such as endotoxin activate macrophages to produce IL-8 and macrophage chemotactic peptides.
Other chemotactic factors are generated during tissue injury as a result of tissue breakdown and death of cells. This is exemplified by the release of proteolytic enzymes following myocardial infarction. These enzymes are released by the dying myocardial cells; they attack collagen and muscle fibers, thus producing chemotactic factors. Neutrophils (and macrophages) then enter the area of infarction and debride the necrotic tissue so that repair can take place.
It appears likely that C5a is involved in the initial recruitment of neutrophils, whereas prolonged recruitment (6–48 h) is due to the production of chemotactic cytokines, such as IL-8.
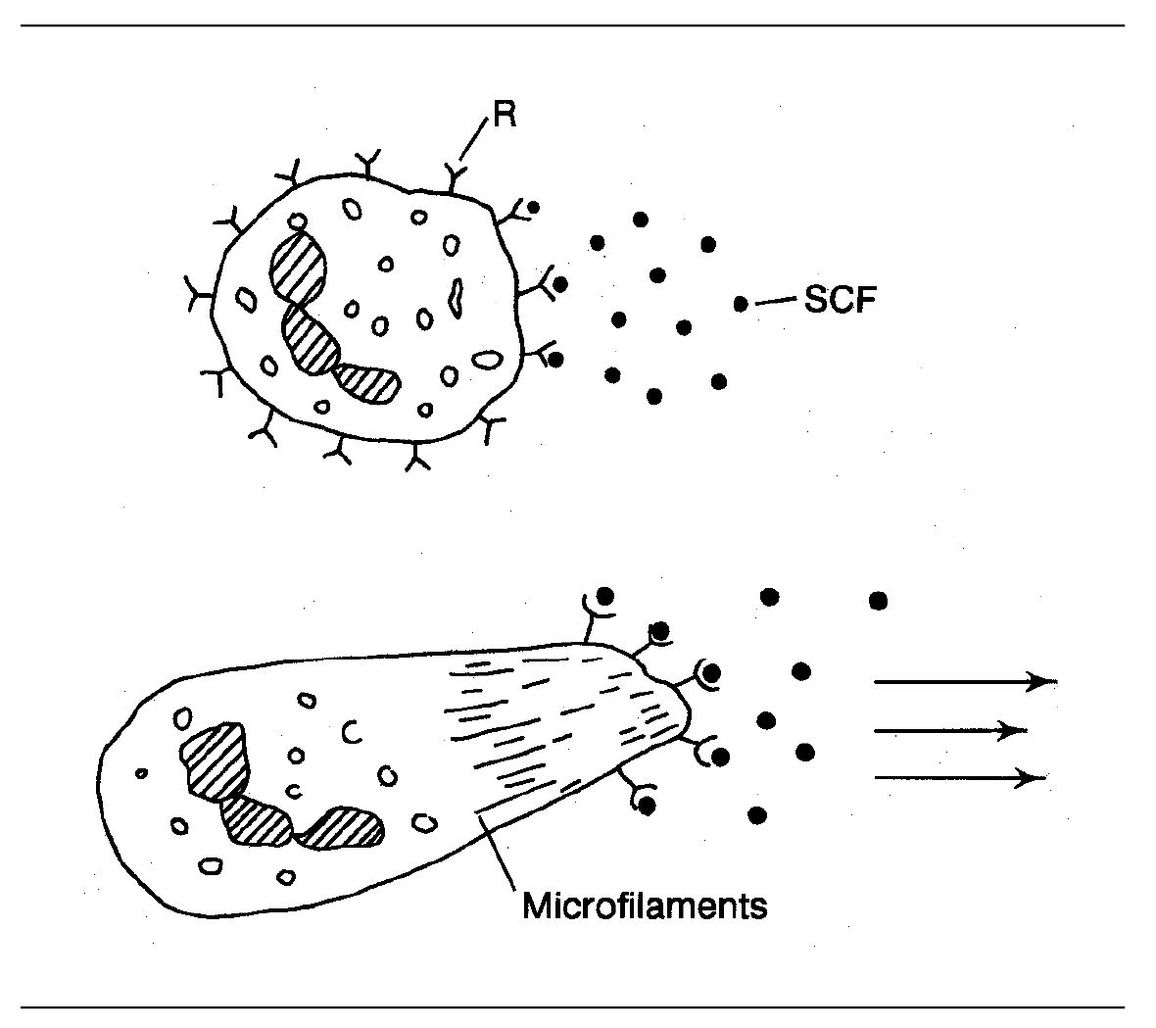
Fig 3-5 Chemotaxis. (R) Receptor for chemotactic factor; (SCF) soluble chemotactic factor.
Chemotactic factors bind to specific receptors on the neutrophil plasma membrane. The attachment of chemotactic factors to these receptor sites results in an influx of calcium ions and the conversion of a proesterase to esterase within the cell. This results in a “flowing” of the cell protoplasm in the direction of the source of the chemotactic factor (Fig 3-5). Locomotion and changes in cell shape are mediated by intracellular microfilaments consisting of actin and myosin. Thus, the neutrophil moves along a concentration gradient of the chemotactic factor until it reaches the agent responsible for chemotaxis. (Driver, follow that factor!)
At this point, the neutrophil is surrounded by chemotactic factors. As there is no longer a concentration gradient to keep the cell moving, the cell stops migrating. (Keep the change!) This keeps the neutrophils in the center of the inflammatory locus.
Neutrophils move rapidly along a chemotactic gradient, whereas macrophages move slowly. This explains why neu-trophils are the first leukocytes to arrive at an injury site. (Of course, they could be just bloodthirsty!)
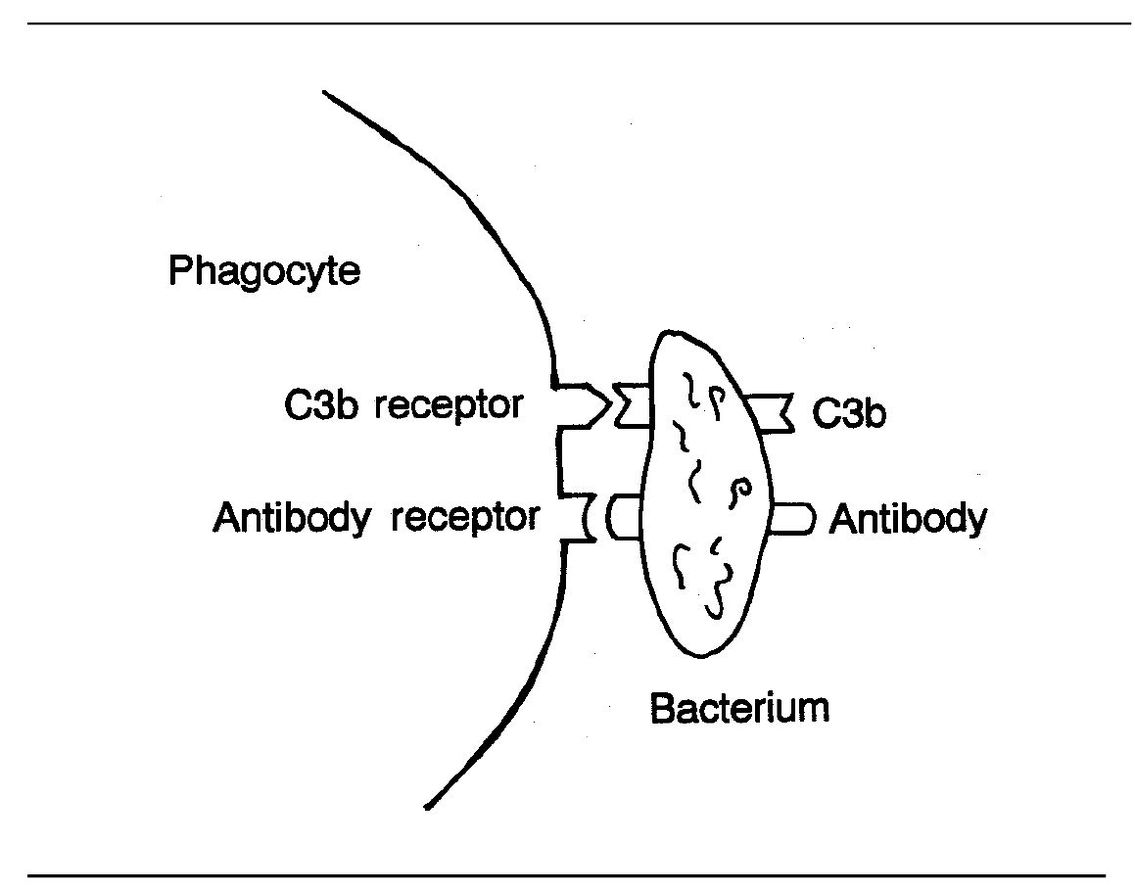
Fig 3-6 Binding of phagocyte to bacterium.
If the agent producing the chemotactic factor can be ingested by the neutrophil (ie, phagocytosed), destruction and digestion of the phagocytosed material may yield breakdown products that also have chemotactic properties.
In fact, neutrophils themselves can be stimulated to release chemotactic factors that attract macrophages. This further increases the number of phagocytic cells attracted to the area of injury, and so the cycle builds. (Another amplification mechanism!)
5. Opsonization
It is difficult for neutrophils to phagocytize most pathogenic bacteria without the aid of serum factors called opsonins. This is because many organisms are resistant to phagocytosis, and here is where opsonins play an important role. In the absence of opsonins, neutrophils must try to trap organisms against tissue constituents such as collagen or fibrin in blood clots and inflammatory exudates.
Opsonins are substances that enhance phagocytosis. One type of opsonins are antibodies that coat the surface of bacteria. In this way, they prepare the organisms for phagocytosis. Once the antibody binds to the bacterial surface, the phagocyte can bind to the Fc fragment of the antibody (see page 99) and ingest the bacterium (Fig 3-6).
Another very important agent in opsonization is complement component C3b. As you will recall, C3b is an opsonic fragment that is fixed to the surfaces of microorganisms during complement activation. Neutrophils and macrophages both have C3b receptors. This receptor acts in conjunction with neutrophil and macrophage receptors for the Fc fragment of lgG. Together these receptors facilitate adherence and phagocytosis of opsonized microorganisms.
Self-test 3-1
- What is our first line of defense against infections?
- From which vessels does emigration of blood leukocytes occur?
- What has to happen before emigration can occur?
- How do phagocytes adhere to endothelial surfaces?
- What are the general properties of chemotactic factors?
- Which complement component is chemotactic factor for neutrophils?
- During chemotaxis, what occurs within phagocytes that enables them to move in a certain direction?
- What are opsonins?
- See if you can match biologic activity with these complement components and split products: C3a, C3b, C5a, C567, C8, C9.
Answers on pages 213–214.
Before considering phagocytosis, let’s recapitulate the events covered so far.
- Injury resulting in vascular responses (vasodilation, increased permeability)
- Slowing of blood flow (stasis)
- Margination of neutrophils and macrophages
- Adhesion (sticking to endothelium)
- Emigration of leukocytes out of venules
- Directed migration to the battlefield (chemotaxis)
- Opsonization
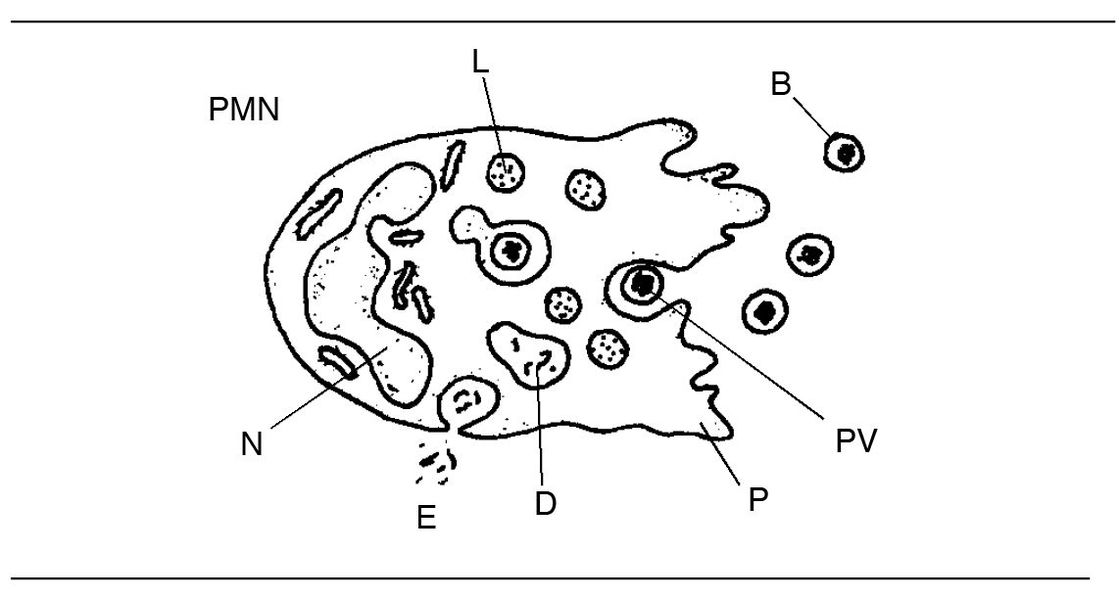
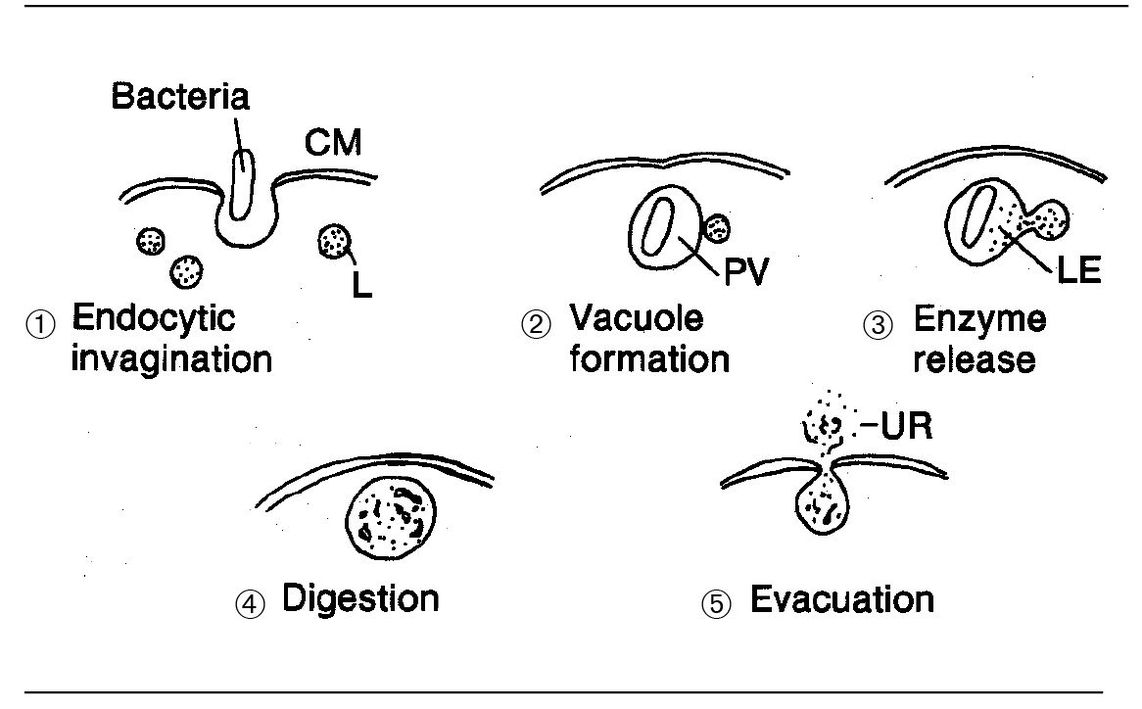
Fig 3-7 Neutrophil structure and phagocytosis. (B) Bacteria; (D) digestion; (E) evacuation of undigestible residue; (L) lysosome; (N) nucleus; (PV) phagocytic vacuole; (P) pseudopodium.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses