Maintenance Considerations in Treatment Planning Implant Restorations
Donald A. Curtis1, Hamilton Le2, and Roy T. Yanase3
1 University of California, San Francisco, California, USA
2 Private Practice in Prosthodontics, Torrance, California, USA
3 Herman Ostrow School of Dentistry, University of Southern California, Los Angeles, California, USA
The concept of maintenance is simple. As clinicians, we have a choice: either we accept the short‐term encumbrance of developing a meaningful in‐office and at‐home maintenance plan, or we face the long‐term prospect of providing an uncomfortable justification for re‐treatment.
Maintenance programs are not sexy. They are not esthetic. There is no wow factor in the dialogue necessary to craft a meaningful maintenance program. However, evidence clearly supports the need for a patient‐specific maintenance program that takes into account patient risk factors. Most importantly, it is the right thing to do.
General considerations
There are few comparative studies to support how clinicians should best maintain dental implants or the tissues supporting implants.1,2 This is not because maintenance is not important, but because few studies have compared protocols specific to the maintenance of dental implants. As a result, the default approach by clinicians has often been to use existing recall and maintenance protocols established for patients with natural dentition. This has worked adequately because debridement benefits the tissues proximal to both dentition and implants. Ideally, maintenance protocols for dental implants would account for the unique biologic processes of tissues around implants as well as the differing prosthetic designs seen in implant‐supported restorations. The focus of this chapter will be clinician‐based considerations and patient‐based responsibilities in the maintenance of dental implants.
Maintenance programs for patients with dental implants are often not considered until restorative care is complete or until the patient has a maintenance issue. This is a mistake. It is important we consider maintenance programs during diagnosis and treatment planning of implant restorations, especially when extensive treatment is planned or when patients present with significant risk factors for peri‐implant disease. Information gathered during diagnosis and treatment planning becomes important when considering the patient’s ability to maintain and clean a planned prosthesis or how the clinician should manage expected functional forces. For example, if the patient was diagnosed as a bruxer, then the developed treatment plan, informed consent, and maintenance protocol would all need to account for controlling, monitoring, and/or dissipating forces. Similarly, if the patient has a history of active or ongoing periodontal disease, maintenance programs and recall patterns should be oriented towards microbial and biofilm management. It is also important that we consider the patient’s compliance and dexterity at the time of treatment, and project 5 years out from the time of treatment on their anticipated dexterity and visual acuity, competence for manipulating cleaning brushes and devices, motivation to maintain healthy tissues, and mobility for office visits.
Maintenance is often seen as the patient’s responsibility, and restorative failures are similarly seen as a result of the patient’s noncompliance. This is an unfortunate perspective. Incentives for the clinician are often prioritized towards satisfying the patient’s immediate esthetic goals and the patient is often fatigued and oriented to completing the lengthy and often involved treatment. Patients are frequently unaware of maintenance needs of their new implant restorations, and may consider their expensive dental implant investment as permanent and “bullet‐proof”. It is easy for both the clinician and patient to be dismissive of maintenance needs. However, maintenance should be considered as a shared responsibility and discussed early during treatment planning. More successful restorative outcomes occur with regular in‐office recalls and follow‐up with reinforcement of at‐home maintenance.1,3 Likewise, evidence shows that when maintenance programs are not followed, biologic and mechanical complications can occur rapidly, asymptomatically, and without obvious early warning signs.1–4 The importance of maintenance programs is well illustrated in a study by Costa et al. in which 80 partially edentulous patients restored with implants and diagnosed with peri‐implant mucositis were monitored to determine how often they progressed to peri‐implantitis over a 5‐year period.3 Patients participating in a maintenance program progressed to peri‐implantitis much less frequently (18%) compared to those not participating in a maintenance program (43.9%).3 Another recent article showed that the incidence of dental implant failure was 90% less in subjects who were enrolled in a maintenance program as opposed to subjects who were not enrolled.5 Recently, numerous articles have pointed to the importance of patient‐specific maintenance programs.4–10 Clearly, certain groups seem more vulnerable to maintenance problems, and maintenance programs are important to improving outcomes. Identifying vulnerable individuals during diagnosis, and developing an appropriate treatment and maintenance plan is important (Figures 3.1 and 3.2).
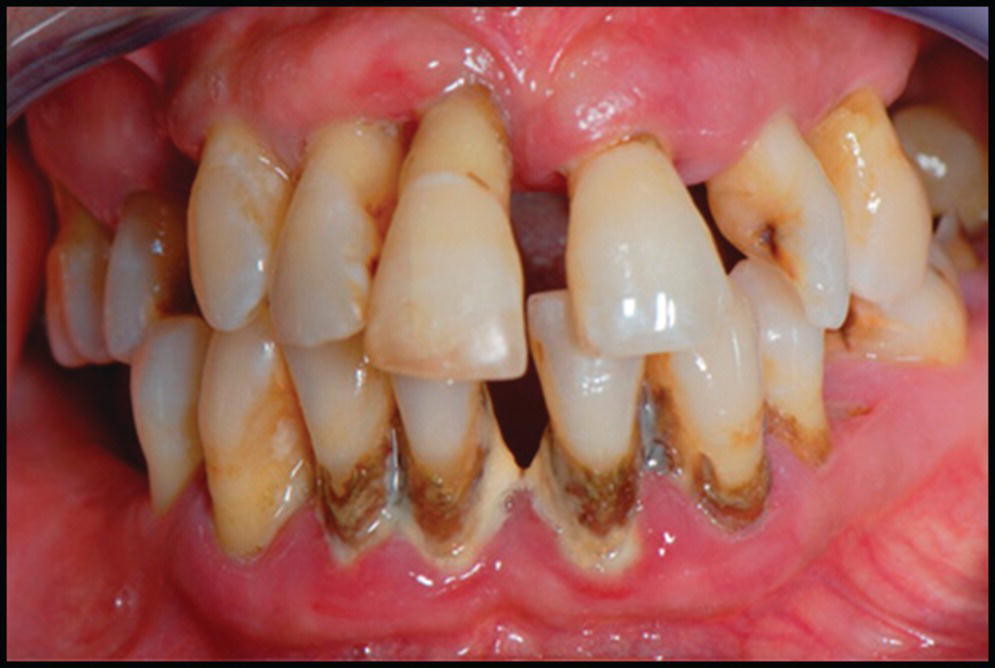
Figure 3.1 In the patient who presents with periodontal disease and poor compliance, the increased risks of implant loss need to be reviewed, and a maintenance program agreed to.1,4,11
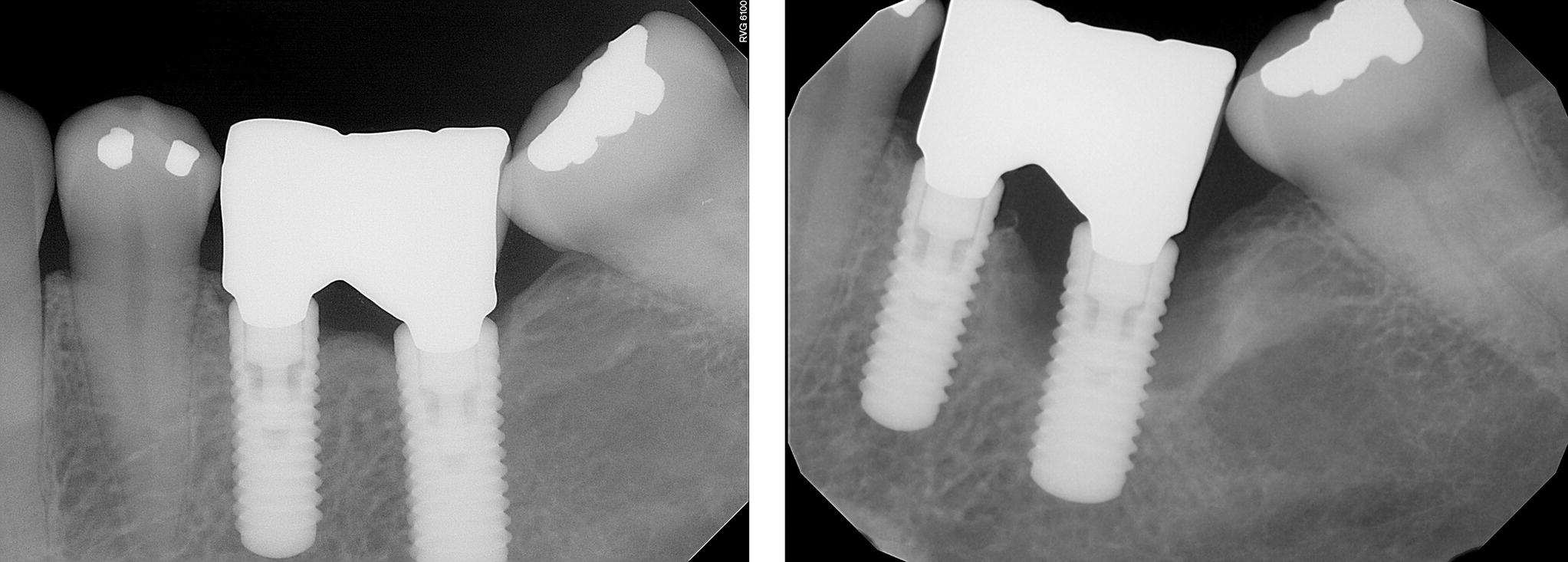
Figure 3.2 At the treatment planning appointment, especially in patients with poor compliance, it is often helpful to show clinical examples of how bone can be lost quickly and asymptomatically around a dental implant. Guiding the patient to articulate a desire for compliance early in treatment can improve outcomes. This is called motivational interviewing and has resulted in improved outcomes in medicine by improving compliance.12
A limitation to establishing meaningful protocols for maintenance of implants is that the etiology of marginal bone loss around dental implants is not completely understood.2 Peri‐implant mucositis around implants is most often thought to be plaque‐related microbial‐based inflammation.13,14 The microbial‐based model and progression of mucositis to peri‐implantitis has been adapted from natural dentition where gingivitis can progress to periodontitis.11 This model makes sense, in that the microbiota in health and disease are similar with teeth and implants; healthy implants are predominately associated with Gram‐positive cocci and rods, and peri‐implantitis is associated with more Gram‐negative anerobic biofilm and a Porphyromonas gingivalis and Fusobacterium nucleatus complex.15 However, there are important differences between implants and teeth in how they react to microbial insults; implants have less of a permucosal seal than teeth, allowing a deeper penetration of bacterial products with more inflammatory cytokines that is more difficult to reverse.2,15,16
It is interesting that comparison studies attempting to reverse mucositis around implants have not been as successful as studies attempting to reverse induced gingivitis around teeth.1 This has led some to question whether peri‐implantitis is entirely explained by a primary infectious process or if the bacteria are opportunistic after the tissue has broken down from nonmicrobial processes.17,18 Bone loss has also been considered to be mediated by strain, with loading causing susceptible bone to microfracture, resulting in a secondary inflammatory process.18 A second contribution to marginal bone loss could also be an aseptic foreign‐body reaction to the implant.18 Because of our incomplete understanding of marginal bone loss, our ability to develop predictable maintenance protocols that target all the known etiologies of marginal bone loss is limited. Yet, even without a complete understanding of the etiologies of marginal bone loss, strong evidence exists that a maintenance program is important, especially with high‐risk groups such as those with a history of periodontal disease.3,4,19
Five‐year implant success rates of dental implants exceed 95%,20 and this has allowed dentists to predictably improve function and comfort for many patients. While this is encouraging, it has also been estimated that over 67 000 dental implants failed in Europe alone in 2008.21 In 2007, replacement costs for implant failures in the US were estimated at over $338 million.21 Our goals are no longer just osseointegration but also for an enduring functional, stable, and esthetic restorative result. This requires a renewed commitment by the profession to promote and reinforce the need for maintenance programs. Maintenance programs can decrease implant failures. It is important that patients are educated to realize the value of maintenance programs and that clinicians use forethought in planning a patient‐specific maintenance program based on patient characteristics and needs, prosthesis design and material, patient compliance, how the patient does in the provisional restoration, and how marginal bone levels respond to the initially developed recall and maintenance program.
Purpose, outline, and definition of terms
The purpose of this chapter is to outline clinician‐ and patient‐based considerations to improve maintenance programs for patients with dental implants. Clinician‐based considerations include: identifying risk factors during diagnosis that impact maintenance, reducing modifiable risk factors, appropriate application of medicaments considered to improve maintenance following delivery, and recommended recall patterns during the maintenance phase. The patient‐based considerations include: recommended at‐home protocols, and suggestions for motivation and reinforcement of at‐home maintenance programs. We will also outline specific recommended maintenance protocols with varied prosthesis designs by using a number of clinical examples. This chapter will include the personal opinions of the authors and a review of the literature relevant to maintenance of implant restorations. An emphasis of the chapter will be a series of publications sponsored by the American College of Prosthodontists, which includes a systematic review on maintenance regimens for patients with implant restorations and a clinical practice guideline (CPG).11,22–24 The CPG includes input from representatives of the American Dental Association, the Academy of General Dentistry, and the American Dental Hygienists Association. Our definition of maintenance includes clinician‐based planning and recommended at‐home interventions designed to decrease mechanical and biologic aftercare needs.
Clinician decisions influencing maintenance programs
Identifying risk factors during diagnosis and treatment planning to develop an appropriate patient‐specific maintenance program
There are many risk factors that can be identified during the history and diagnosis stage of treatment that are relevant to developing a maintenance program. Identifying patients with a history of bruxism, periodontal disease or refractory periodontitis, smoking, diabetes, use of bisphosphonates, or radiation treatment are all relevant to the risk of biologic and/or mechanical complications.25–68 An early dialogue with the patient regarding anticipated maintenance needs and recall recommendations will likely result in improved patient compliance once treatment is completed.
During the diagnosis and treatment‐planning phase, it is important to assess potential occlusal and parafunctional forces developed by the patient. This information can be learned through patient history, but also by carefully evaluating the patient’s existing dentition. In a 10‐year retrospective study of 397 patients being evaluated for mechanical and biologic complications, Wittneben et al.20 showed a strong association between attrition in the pre‐treatment evaluation of dentition and incidence of later mechanical complications of implant restorations. Specifically, with no, localized, or generalized signs of attrition, the incidence of porcelain fracture increased from 10.9%, to 21.9%, and 26.9%, respectively. This means that the pre‐treatment evaluation of attrition may be a valuable indicator in informed consent, material selection, number of implants used, and in developing a patient‐specific maintenance protocol. In bruxers, the use of a nightguard and more frequent recalls to monitor screw loosening should be considered. Wittneben et al.20 also showed that multiple‐unit splinted crowns had a higher risk of porcelain chipping than single units. Individuals with higher force should have a treatment plan with more implants, more detailed informed consent, and more regular recall patterns to monitor potential mechanical complications. In a retrospective study of 152 patients, Kinsel and Lin showed that patients with bruxism had seven times the incidence of porcelain fracture as compared to non‐bruxers.25 The authors also showed that patients had fewer complications when a nightguard was used or when the patient had canine‐protected occlusion in their restorations. Clearly, there is evidence for the need to complete a thorough history of potential bruxing habits, to evaluate the dentition carefully for attrition, and to appropriately plan the use of materials, occlusion, and potential use of a nightguard to help maintain the designed implant prosthesis (Figure 3.3).
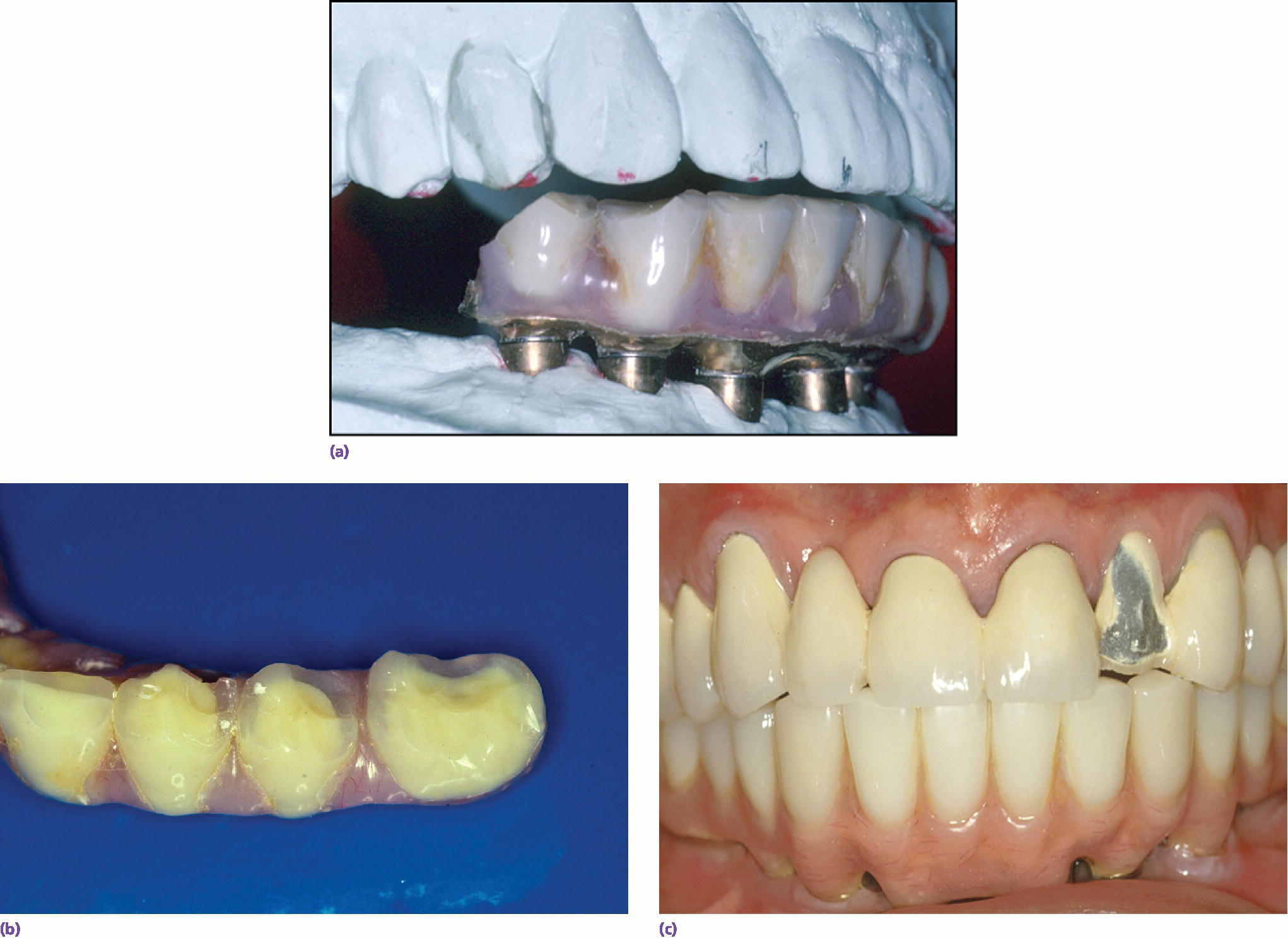
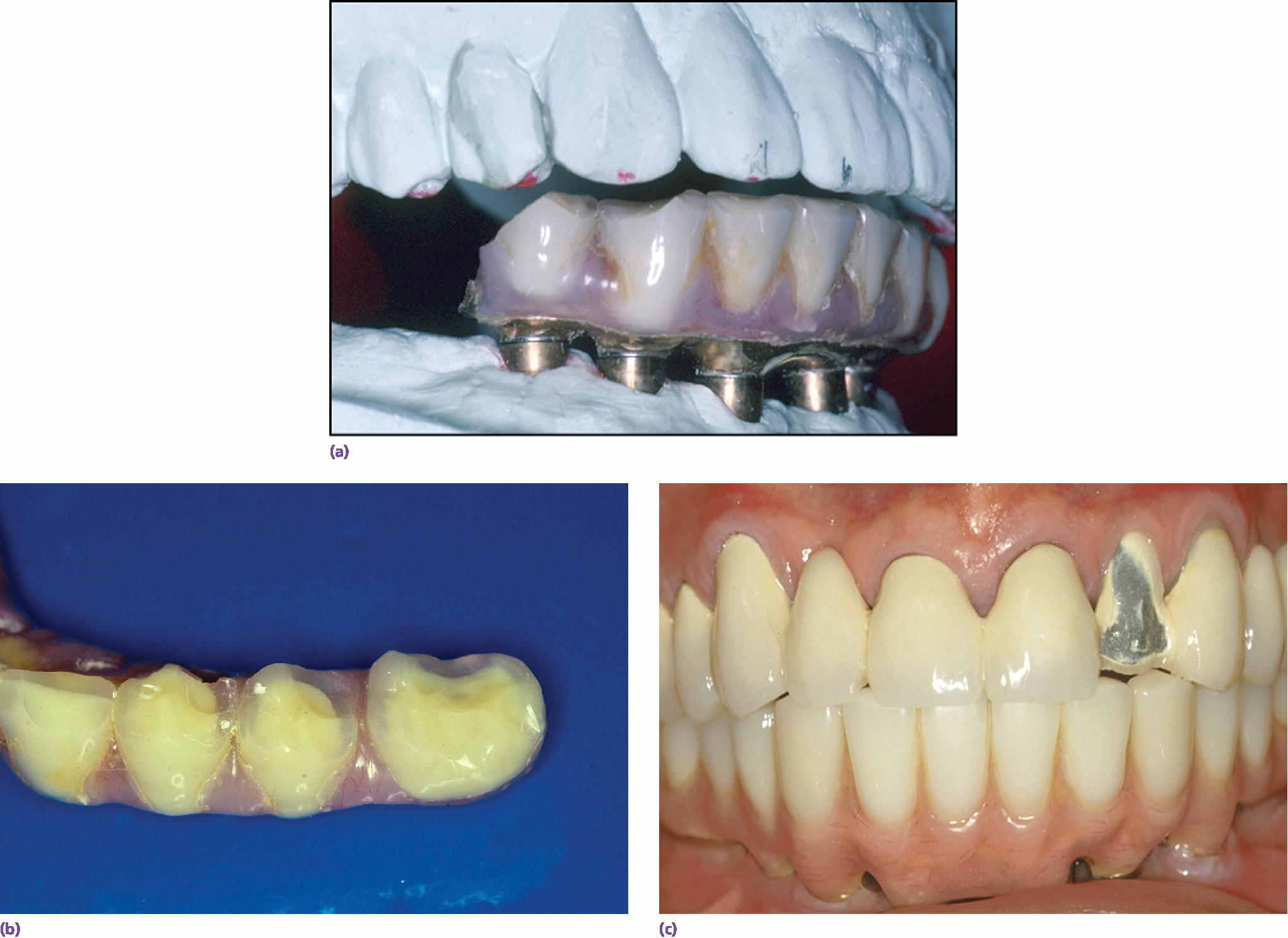
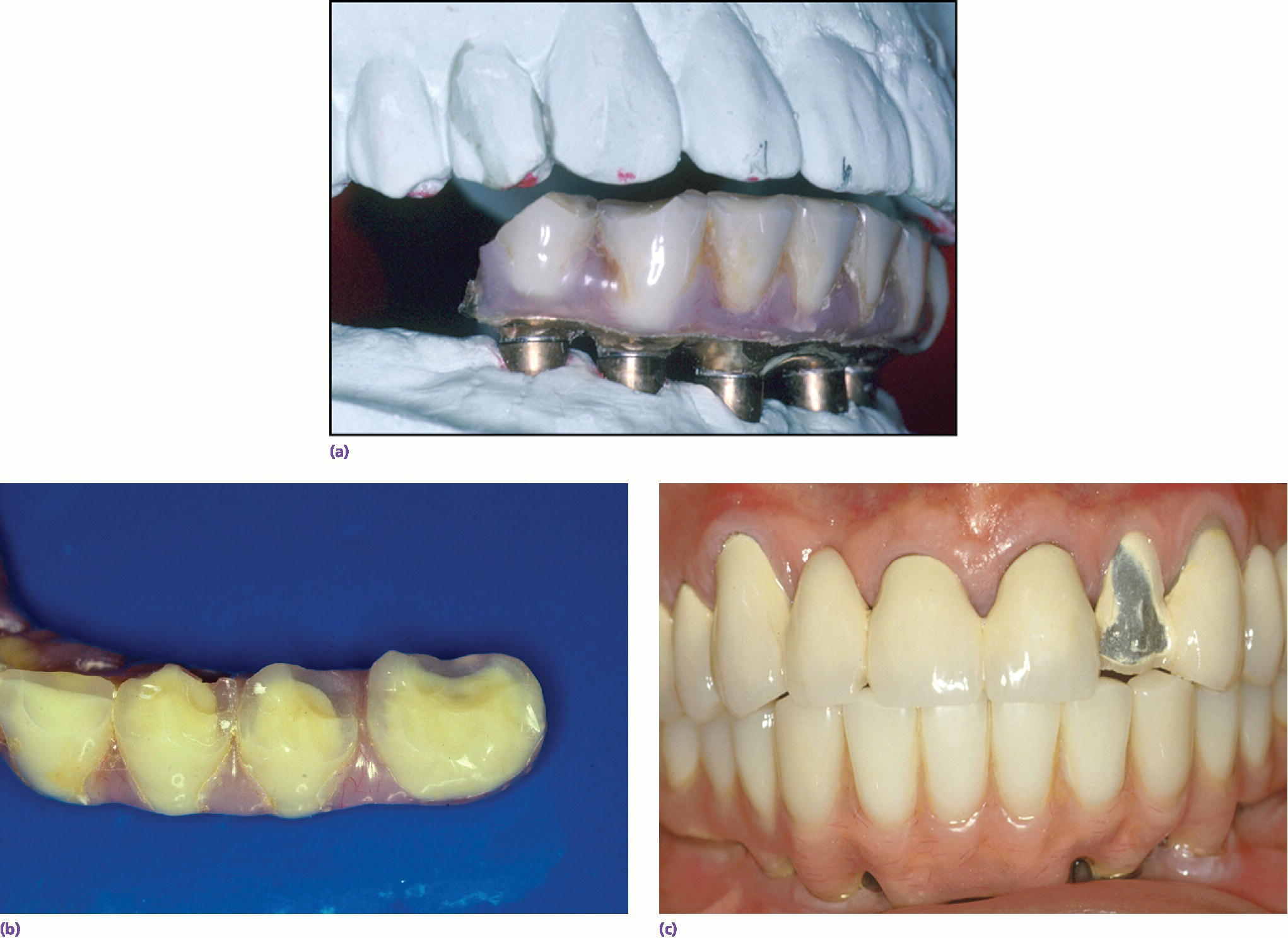
Figure 3.3 (a–c) Maintenance needs are expected with implant restorations. In a recent systematic review of technical and biologic complications over a 10‐year period, only 8.6% of prostheses did not have complications.52 Both the patient and clinician need to prepare for this potential.52 The most common biologic complications were hypertrophy or hyperplasia of tissue and inflammation under the prosthesis. The most common technical complications included chipping/fracture of veneering material, screw loosening, screw fracture, fabrication of new opposing prostheses, replacement resin teeth, wear of resin teeth, framework fracture, and patient dissatisfaction.52
The relationship between overloading and bone loss is not completely understood. A review of clinical and animal studies was recently completed by Naert et al.36 who concluded that, in the absence of peri‐implant inflammation, overload did not significantly contribute to peri‐implant bone loss. They did find out, however, that with high loads and poor oral hygiene in susceptible patients, bone loss may occur.
Patients with a history of periodontal disease are particularly vulnerable to peri‐implant disease, making a maintenance program especially important to consider.1,6,7 Unfortunately, the criteria to establish a diagnosis of peri‐implant disease remain controversial and are inconsistently applied by investigators, so it is not surprising that there is a wide variation in reported incidence of peri‐implant disease.18 The incidence of peri‐implant disease varies depending on the group studied and the disease criteria, but it has been conservatively estimated to occur in 10% of implants and 20% of patients during a 5–10‐year period.18 Other estimates from a recent systematic review estimate that peri‐implant mucositis occurs in 43% of patients, and peri‐implantitis in 22% of patients.1 In contrast, Abrektsson et al.18 suggest, in a review of three modern implant brands with 10‐year or longer follow‐up data, that the diagnostic value of bleeding on probing may be less valuable than originally thought. His review showed that the incidence of peri‐implantitis was less than 3% during a 7–16‐year period.18 Interestingly, and in contrast to Albrektsson et al., a recent systematic review and expert consensus statement found that bleeding on probing was considered the most consistent way to diagnose peri‐implant disease, but that changes in probing depth also should also be considered.1 It has also been suggested that using a light probing force (0.25 N) will minimize damage to the supporting tissues.1,51
The literature on maintenance programs and monitoring of peri‐implant disease is often based on convenience samples that are usually of limited size, often from university clinics, variable in follow‐up time, and predominantly retrospective.6,13,26 Therefore, the patient populations studied are often heterogeneous and not representative, which makes it difficult to identify trends. The clinical measures used to establish a diagnosis of peri‐implant mucositis or peri‐implantitis have not been consistent among studies, which makes combining data in systematic reviews or meta‐analyses difficult.1,13 Furthermore, comparative clinical studies of definitive maintenance protocols are lacking.28 Therefore, the progression of peri‐implant disease and the impact of specific homecare and recall regimens are not well understood,13 which makes establishing the efficacy of maintenance protocols and recommendations problematic.
Several studies, including a recent systematic review, have shown no difference in implant survival rate in patients with periodontitis and those without periodontitis if the patient is enrolled in a maintenance program.28,29 However, patients with a history of periodontitis did have more marginal bone loss around their implants, and peri‐implantitis was more prevalent in patients with periodontitis, which would have implications in long‐term follow‐up.27 In a retrospective study of 97 patients, Hardt et al. evaluated bone loss and implant loss in patients with or without periodontal disease in their natural dentition.4 The investigators determined that 64% of patients with periodontal disease on natural dentition had peri‐implant bone loss of more than 2 mm compared to 24% of the nonperiodontal patients. In a prospective 5‐year randomized trial of 51 patients with a total of 149 implants, Wennstrom et al. concluded that, while in a maintenance program, patient’s bone loss was minimal, even for those with a background of periodontal disease.19 Conversely, Monje et al. showed the risk ratio for implant failure in patients with generalized aggressive periodontitis to be 4.0, when compared to healthy patients.17 In a systematic review of patient history of periodontitis on implant success, Ramanauskaite et al. concluded that survival rates among those with a history of periodontitis are comparable with nonperiodontitis patients, but that there is a trend for more marginal bone loss in periodontitis patients.27 It appears from the literature that those patients with a history of periodontal disease are at a significant short‐term potential for increased bone loss and a long‐term potential for increased implant loss when compared to those without a history of periodontal disease. Maintenance plans have been shown to partially mitigate this trend.29
Diabetes is a risk factor for biologic complications that can potentially adversely impact outcomes, especially if coupled with other risk factors such as periodontitis, poor oral hygiene or smoking.34,35,39 Significant factors include the amount of time the patient has been diabetic and if the HbA1C level is above 7%.35 The mechanism behind the increased incidence of peri‐implantitis and marginal bone loss in diabetics is related to the disruption of collagen.35,39 Smoking may also potentiate implant failure in diabetics.38 Although a recent systematic review did not find an association between type 2 diabetes and implant failure, it did find a difference between marginal bone loss and a diabetes diagnosis.35 The mechanism of bone loss is not entirely understood, but in susceptible diabetic patients, it includes the disruption of collagen formation and integrity.39
Decreasing modifiable risk factors to improve maintenance of implant restorations
The most obvious modifiable risk factor for implant success is minimizing or eliminating smoking habits. Tobacco products contribute significantly to periodontal disease and smoking is an avoidable risk factor for biologic complications with dental implants.38–40 Patients who are heavy smokers, defined by smoking more than 30 cigarettes a day, have increased odds of implant loss compared to those who smoke fewer cigarettes.39 Smoking also increases the risk of postoperative infections, and may result in epigenetic changes that increase risk for implant failure by downregulating of osteopontin and type II collagen.38,39 In patients under a regular maintenance program, the impact of smoking appears to be lessened.29 However, poor oral hygiene has been shown to result in more bone loss in smokers than in nonsmokers.40
Cemented restorations can be a risk for peri‐implant disease, both because of residual cement and because of retained plaque at the subgingival margins. Cemented restorations should be supragingival if possible, and specific techniques should be used to limit cement into the sulcus. In a double‐blind prospective study of 29 patients, Heitz‐Mayfield et al.37 evaluated the baseline soft‐tissue measures in patients with mucositis by measuring bleeding on probing, pocket depth, suppuration, and plaque. Debridement was then completed on all 29 patients and follow‐up provided after 1 month and 3 months. The authors showed that patients with cemented restoration margins that were subgingival demonstrated less improvement in probing depth measurements than in patients with supragingival restorations. Planning adequately for a screw‐retained prosthesis should be a goal in treatment planning, and when cementing is necessary, having margins supragingival should be used to improve soft‐tissue responses. Clinicians can also consider pre‐cementation procedures to decrease extrusion of cement.
There are modifiable risk factors in material selection as well. If there are planned posterior opposing implant restorations, it is advisable to be especially careful in material selection. The use of metal occlusals, the use of a nightguard, and the goal of anterior disclusion are favorable in order to decrease mechanical complications.25 As the esthetics of monolithic zirconia restorations improves, more of these restorations are likely to be utilized as long as wear of titanium fixtures from the zirconia can be minimized.
Stay updated, free dental videos. Join our Telegram channel

VIDEdental - Online dental courses


